Chemistry ✿ required practicals PAPER 2
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
![<p>Some students investigated the effect <strong>of temperature</strong> on the <u>rate of reaction.</u> </p><p>The students reacted <strong>sodium thiosulfate</strong> solution with <strong>hydrochloric acid.</strong></p><p>Describe a method the students should use to <strong><em>produce accurate results [6]</em></strong></p>](https://knowt-user-attachments.s3.amazonaws.com/6b64a5ec-c943-4adf-9869-936016bd267d.png)
Some students investigated the effect of temperature on the rate of reaction.
The students reacted sodium thiosulfate solution with hydrochloric acid.
Describe a method the students should use to produce accurate results [6]
pour 10cm³ of sodium thiosulfate solution into a conical flask
add 10cm³ dilute hydrochloric acid into conical flask and place it on a black cross and start a stopwatch
measure temperature of the solution
stop stopwatch when cross has disappeared
measure temperature at the end of the reaction
repeat experiment at different temperatures
![<p>Some students investigated the effect <strong>of concentration </strong>on the <u>rate of reaction.</u></p><p>A student investigated the reaction between <strong>magnesium</strong> and <strong>hydrochloric acid. </strong><br>Describe a method the students should use to <strong><em>produce accurate results [6]</em></strong></p>](https://knowt-user-attachments.s3.amazonaws.com/ca6c5ead-3e68-4035-a598-f0d526b3d96f.png)
Some students investigated the effect of concentration on the rate of reaction.
A student investigated the reaction between magnesium and hydrochloric acid.
Describe a method the students should use to produce accurate results [6]
set up a conical flask containing 50cm³ of 1mol/dm³ hydrochloric acid
have bung and delivery tube attached to gas syringe prepared
add a 3cm piece of magnesium ribbon
quickly fit bung on conical flask and start a stopwatch
record the measurement of gas produced every 10 seconds
repeat experiment with 2 and 3 mol/dm³ hydrochloric acid
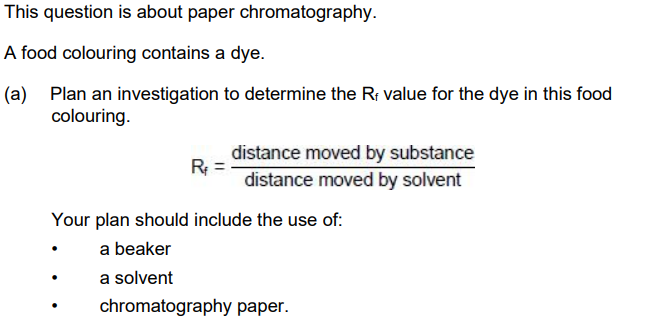
Plan an investigation to determine the Rf value for the dye in this food colouring.
Your plan should include the use of
a beaker
a solvent
chromatography paper
draw pencil start line on chromatography paper
using a capillary tube, add a dot of the 4 known inks and the unknown substance
add water inside a beaker, make sure it isn’t too much that the pencil line is under the water
attach a glass rod to the filter paper using tape and lower it in the beaker, making sure the sides do not touch the walls
add a lid onto the beaker
after 30 minutes remove the chromatogram, let it dry and take measurements needed to calculate the Rf values using the equation
Describe a method to determine the mass of dissolved solids in a 100 cm³ sample of river water [4]
weigh evaporating dish using scale
measure 100cm³ of river water using measuring tube and pour water into evaporating dish
use Bunsen burner to evaporate water until dry
weigh evaporating dish again with remaining soils
Explain how paper chromatography separates substances. [2]
solvent moves through paper
and carries substances different distances
due to their attraction for paper and solvent
random errors
uncontrollable changes in an experiment
systematic errors
faulty equipment or method
zero error
when scale is not set to zero properly
what is the independent variable in chromatography?
solvent (water)
what is the dependent variable in chromatography?
solubility of dyes/ inks
what are 2 control variables in chromatography?
amount of solvent
temperature of solvent