Endocrinology: Testosterone Replacement for Hypogonadism (Xavioer)
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
What is testosterone replacement therapy primarily used to treat?
Hypogonadism in men
What are the characteristics of primary hypogonadism?
Low testosterone; elevated gonadotropin
What are the characteristics of secondary hypogonadism?
Low testosterone; low-normal gonadotropin
What are the characteristics of mixed hypogonadism?
Low testosterone; variable gonadotropin levels
When does the Endocrine Society recommend testosterone therapy?
For men with symptomatic testosterone deficiency to maintain secondary sex characteristics and correct hypogonadism symptoms.
What is the AUA's recommended cut-off for diagnosing low testosterone?
A total testosterone level below 300 ng/dL
How many total testosterone measurements are needed for diagnosing low testosterone?
Two measurements taken on separate occasions, both in the early morning
In which conditions should total testosterone be measured, even without symptoms of deficiency?
Unexplained anemia, bone density loss, diabetes, exposure to chemotherapy or testicular radiation, HIV/AIDS, chronic narcotic or corticosteroid use, male infertility, and pituitary dysfunction
Why are oral testosterone products not recommended as a first-line agent?
Due to the risk of hepatotoxicity
What is the initial dose of oral testosterone undecanoate (Jatenzo®)?
237 mg twice daily (BID) with food
When should the dose of Jatenzo® be adjusted?
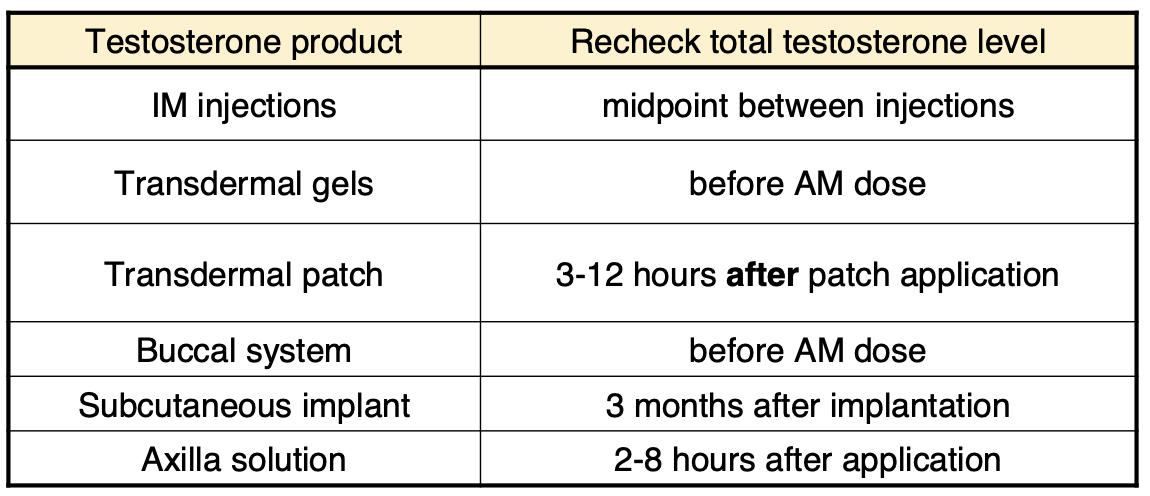
Based on the AM testosterone level, taken 6 hours after the morning dose, 1 week after starting treatment
What are the available capsule strengths of Jatenzo®?
158 mg, 198 mg, and 237 mg
Is Jatenzo® recommended for age-related hypogonadism?
No
What is a side effect of intramuscular testosterone injections due to supraphysiologic serum concentrations?
Mood swings
What is the black box warning (BBW) for testosterone undecanoate injections?
Pulmonary oil microembolism (POME) reactions, including cough, shortness of breath (SOB), chest pain, and anaphylaxis
How is testosterone undecanoate available?
Only through a REMS (Risk Evaluation and Mitigation Strategy) program
How long should patients be monitored after a testosterone undecanoate injection?
For at least 30 minutes
What is the dosing frequency for subcutaneous testosterone enanthate?
Once-weekly subcutaneous injection
Are testosterone injections recommended for age-related hypogonadism?
No
How often should buccal testosterone be applied?
Twice daily, every 12 hours
How should buccal testosterone be applied?
Press against the gum for 30 seconds and rotate sides with each application
What is the strength of testosterone buccal tablets?
30 mg
What are common side effects of buccal testosterone?
Gum/mouth pain, irritation, and taste disturbance
How often should nasal testosterone (Natesto) be used?
One pump per nostril, three times daily
How much testosterone is delivered per actuation of Natesto?
5.5 mg per actuation
What are common side effects (ADRs) of nasal testosterone?
Nasal irritation, nosebleeds, rhinorrhea, and nasopharyngitis
What are the available dosages for testosterone cypionate, enanthate, and undecanoate?
Cypionate: 100 mg/mL and 200 mg/mL
Enanthate: 200 mg/mL
Undecanoate: 750 mg/3 mL
How long should you wait before reapplying the patch to the same site?
7 days
Where should testosterone gel (Testim) be applied?
Shoulders or upper arms only
What is the black box warning (BBW) for testosterone gel?
Secondary exposure — cover the application site with clothing to prevent transfer
How often should you rotate the application site for a testosterone patch?
Rotate the site with a 7-day interval before reapplying to the same area
Where should the transdermal spray be applied?
To the front or inner thighs only
Where should transdermal testosterone solution be applied?
To the armpit (axilla) only
What schedule are testosterone products classified as?
Schedule-III controlled substances
Who are testosterone products contraindicated for?
Men with breast or prostate cancer, and pregnant or breastfeeding women (or those who may become pregnant)
What is the black box warning (BBW) for oral testosterone undecanoate and subQ enanthate?
Increased blood pressure (BP)
In what cases should testosterone products be avoided due to caution or warnings?
In men with recent myocardial infarction (MI) or stroke within the past 6 months, and men with severe lower urinary tract symptoms (LUTS)
What is a common fluid-related adverse effect of testosterone products?
Fluid retention
What is the effect of testosterone products on hematocrit?
They can increase hematocrit, leading to polycythemia
In terms of metabolic effects, what will testosterone increase?
Insulin sensitivity
Glycemic control
Lean body mass
Muscle mass
Triglycerides; ↓ HDL-C
In terms of metabolic effects, what will testosterone decrease?
Subcutaneous fat
What prostate-specific antigen (PSA) level indicates that testosterone should not be initiated?
PSA >4 ng/mL, or >3 ng/mL if at high risk for prostate cancer
What hematocrit level indicates that testosterone should not be initiated?
Hematocrit >48%, or >50% in high-altitude areas
What ongoing monitoring is required once testosterone replacement therapy is stable?
Testosterone levels, CBC (hematocrit), PSA*, liver function, lipids, symptoms of low testosterone, and adverse effects of medication
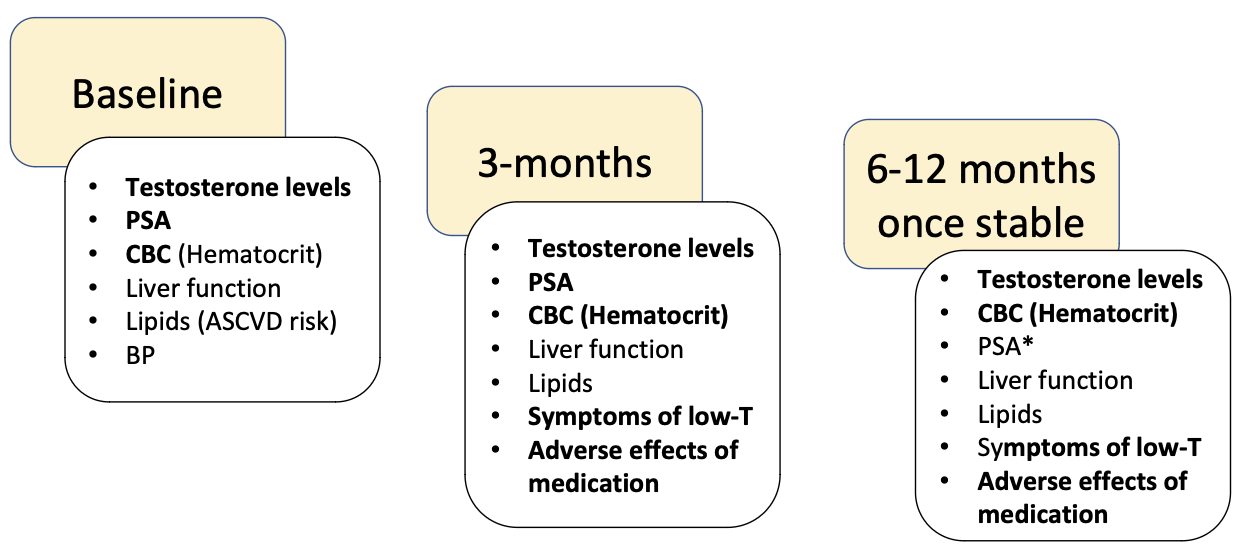
When should testosterone therapy be discontinued based on PSA levels?
Discontinue if PSA increases by more than 1.4 ng/mL from baseline or if PSA is >4.0 ng/mL
What hematocrit level indicates that testosterone therapy should be discontinued?
Hematocrit >54%
What testosterone concentration level indicates discontinuation for products like Striant and Natesto?
Testosterone concentration >1050 ng/dL
When should you recheck testosterone levels after initiating?
See image