Adrenal glands- androgens and the medulla
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
adrenal androgens
Small amounts synthesised in zona reticularis
DHEA and androstenedione- in females cause growth of pubic and axillary (armpit) hair, female libido?
What is congenital adrenal hyperplasia
Enlarged adrenal glands, most commonly caused by 21 hydroxylase deficiency
Symptoms:
dehydration, salt loss, weakness
female: male genitalia, hirsutism
male: precocious puberty
treatment = corticosteroid replacement
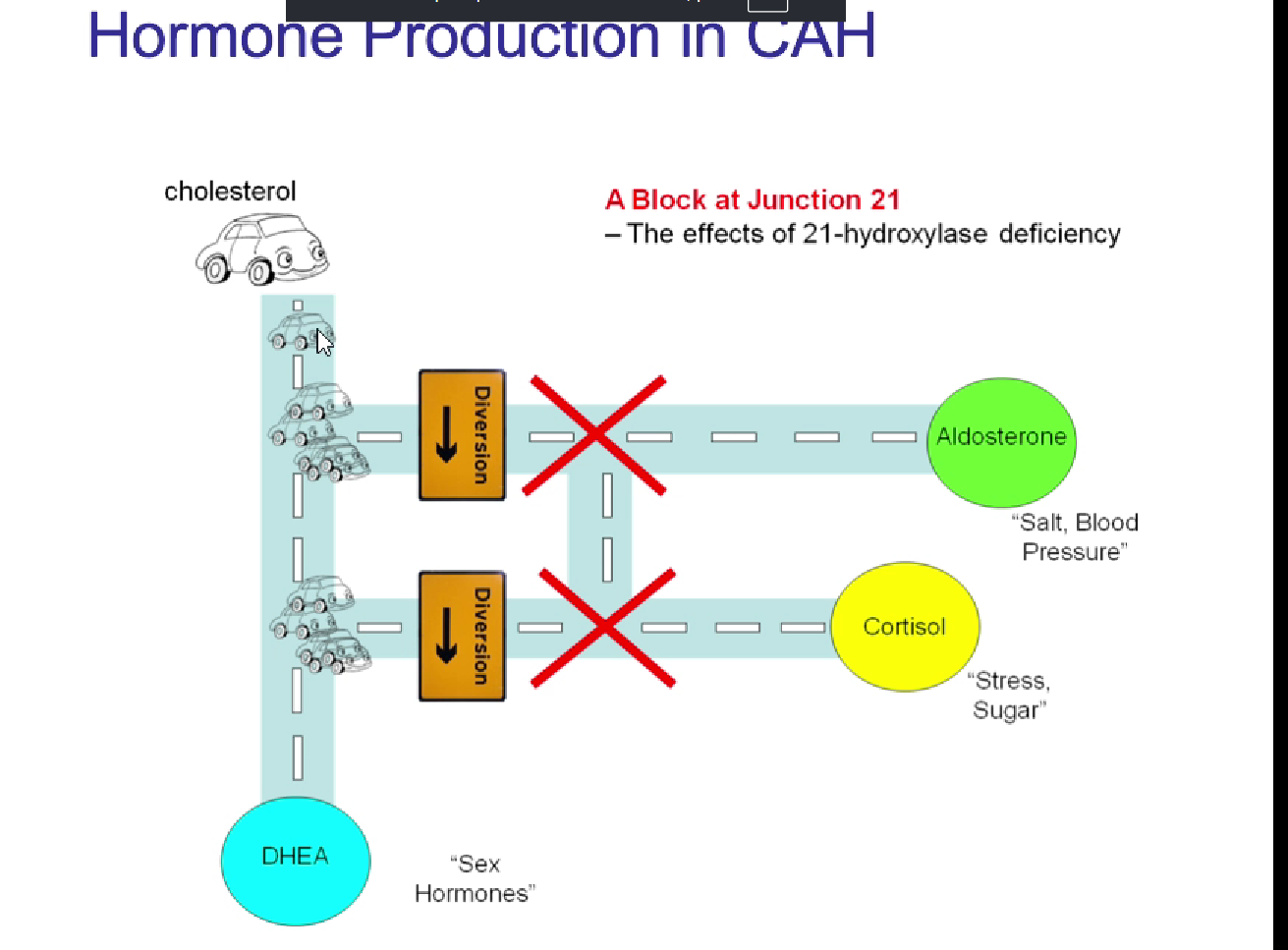
CAH mechanism
reduced glucocorticoid and mineralocorticoid production
reduced -ve feedback —> increased ACTH —> adrenal hyperlasia
leading to excessive production of adrenal androgens
outline the spirogenic pathway from 21 hydroxylase deficiency to androgen excess
lose CYP21 in hydroxylase deficiency, stops production of cortisol (and mineralocorticoids), no -ve
anterior pituitary keeps producing ACTH until adrenal glands start producing DHEA which enlarges adrenal glands
more DHEA = more androgens
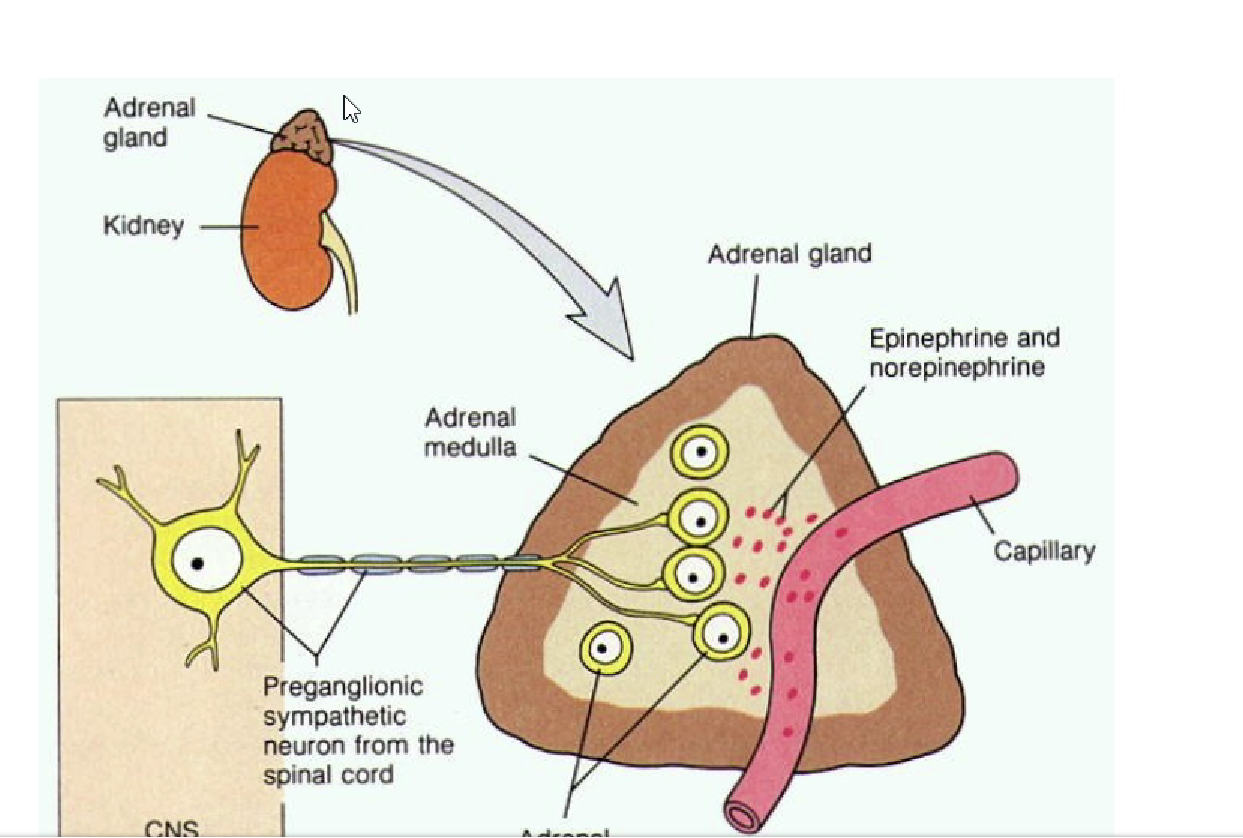
give an overview of the adrenal medulla
part of the autonomic NS
specialised ganglia supplied by sympathetic preganglionic neurons
synthesises catecholamines
main site for adrenaline synthesis
not essential for life!
Outline the anatomy of the medulla
Alpha and beta androgenic receptors
Chromaffin cells produce adrenaline and noradrenaline
Blood supply = past cortex to the medulla
PNMT
phenylethanolamine N-methyltransferase
DONT REMEMBER THAT
an enzyme that converted noradrenaline to adrenaline
Outline catecholamine synthesis
OVERALL: tyrosine —> noradrenaline
Tyrosine hydroxylase catalyses the rate limiting step: tyrosin —> L-DOPA
L-DOPA —> dopamine
Dopamine —> noradrenaline (dopamine beta-hydroxylase w/in the synaptic vessels
noradrenaline —> adrenaline w/PNMT
production of more adrenaline than noradrenaline
outline the relationship between cortisol and catecholamines
high cortisol —> high concentration in capillary blood
activates PNMT molecules, meaning more molecules w/noradrenaline will have adrenaline
outline catecholamine storage and regulation
catecholamines transported to synaptic vessels using specialised transporter after synthesis
vesicles protect hormone from degredation, ensure control can be maintained over how much is released
high catecholamine levels w/in the nerve terminal inhibit rate limiting step (tyrosine hydroxylase, -ve feedback)
high level of stimulation from nerves stimulates tyrosine hydroxylase
outline catecholamine release
cortisol doesn’t care how much adrenaline there is, not -ve
adrenaline broken down quickly- VMA = waste product excreted in urine
Systemic effects of adrenaline
differential receptor sensitivity
alpha: Adrenaline < noradrenaline
beta: adrenaline > > noradrenaline
adrenal medulla activity- producing lots of adrenaline, primarily activating beta receptors
central nervous system effects of adrenaline
increased alertness/arousal
increased anxiety
increased muscle tremor
adrenaline effects: cvs and lungs
Cardiovascular system
heart rate increases, force of contraction
vasodilation
bronchodilation of respiratory system
adrenaline effects: metabolism
glycogen breakdown in muscle
increases blood glucose
adrenaline effects: hepatic
increased glycogenolysis/gluconeogenesis
increases blood glucose
adrenaline effects: adipose tissue
mobilisation of free fatty acids
increases blood glucose
phaeochromocytoma
excess catecholamines due to tumour of chromaffin cells (in adrenal medulla)
-→ chronic over-secretion or dramatic episodes from a sudden stressor (exercise, rapid postural changes)
phaeochromocytoma symptoms
episodes of very high BP
sudden, severe headache
palpitations, chest pain
pallor of skin, sweating
anxiousness
phaeochromocytoma treatment
surgery AFTER MANAGING potential catecholamine crisis risk (cardiac arrest)
alpha blockers to prevent vasoconstriction
surgery
then beta blockers after surgery to minimise cardiac stimulation
no surgery option = anti-hypertensive drugs
Which hormone is most affected in cushing’s syndrome, and is it in excess or deficit?
Cortisol in excess
Extra: some forms of Cushing syndrome caused by Cushing disease or an ectopic tumor —> excess ACTH
What is the most common cause of Cushing’s syndrome?
Cushing’s disease, a tumour on the adrenal gland causing an overproduction of glucocorticoids including cortisol
What are other causes of cushing’s syndrome?
Long term stress
Long-term glucocorticoid drug use for autoimmune conditions
An ectopic tumor anywhere in the body that overproduces ACTH
What medications might a person with cushing’s syndrome be taking long-term?
Steroid medications
Outline the symptoms of Cushing’s and their causes
osteoporosis, broken bones
high cortisol reduces calcium absorption and increases calcium excretion
muscle weakness, skin fragility, bruising
high cortisol leads to the catabolism of muscle and skin proteins for gluconeogenesis, leading to muscle loss and fragile skin
fat redistribution
excess cortisol leads to fat loss from the extremities and fat gain to the trunk and face
skin infections
high cortisol levels lead to immunosuppression, leading to increased infection risk and poor wound healing
‘feeling out of sorts’
excess cortisol leads to changes in mood and cognition
Why is an elevated urine glucose level consistent with Cushing’s?
High cortisol levels —> increased plasma glucose level caused by an increase in hepatic gluconeogenesis
kidneys unable to filter out all the excess glucose in the blood
leads to excretion of glucose in urine
Why is an elevated BP consistent with Cushing’s?
high cortisol levels means that there are fewer cortisol receptors relative to cortisol molecules, causing cortisol to start binding to mineralocorticoid receptors in the kidneys
mineralocorticoid receptors increase sodium reabsorption in the kidneys, which in turn —> high blood pressure
an increase in cortisol leads to an increase in cholesterol production, as cortisol is a cholesterol derivative
this potentially —> higher levels of aldosterone produciton, as there is too much cholesterol and not enough cortisol-specific enzymes to convert all of the cholesterol to cortisol?
what is phaeochromocytoma
a tumor of the chromaffin cells in the medulla of the adrenal glands that causes an overproduction of catecholamines including adrenaline and noradrenaline
non-specific: can be adrenaline, noradrenaline or both
how is phaeochromocytoma diagnosed?
through BP measurement to diagnose high BO
urine test to measure VMA levels produced by the breakdown of adrenaline
A scan using tracer to look for the tumor itself
Why is elevated heart rate and BP consistent w/phaeochromocytoma?
catecholamines —> increase in HR through acting upon alpha and beta receptors, which increase the speed and force with which the heart contracts
how can phaeochromocytoma be treated?
increased activation of alpha receptors through excess adrenaline —> increased risk of catecholamine crisis including a cardiac arrest
treatment prevents a catecholamine crisis w/alpha blockers
after this prevention, surgery used to remove tumors
beta blockers if surgery not an option or to prevent additional complications
what are alpha and beta receptors?
g-protein coupled receptors that are the targets of catecholamines and drugs such as beta blockers