electrons and bonding
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
what are shells
the energy levels/the regions around the nucleus where electrons are most likely to be
what is the formula to work out number of electrons in a shell - n=the shell number
2n² - 2x(n²)
how many electrons can the first 4 shells fit
1 - 2
2 - 8
3 - 18
4 - 32
what is an orbital
a region around the nucleus where 2 electrons can be found (with opposite spins)
what are the 4 types of orbital
S, P, D, F
what shape is an s-orbital
a sphere
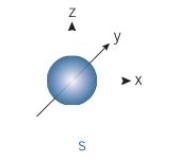
how many s-orbitals are there per shell
1
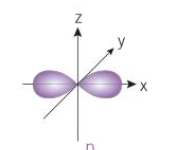
what shape is a p-orbital
a dumbbell shape
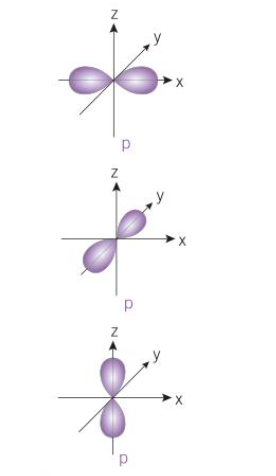
how many p-orbitals per shell
3 - one for each direction (only from shell 2 onwards)
how many d-orbitals per shell
5 (only from shell 3 onwards)
how many f-orbitals per shell
7 (only from shell 4 onwards)
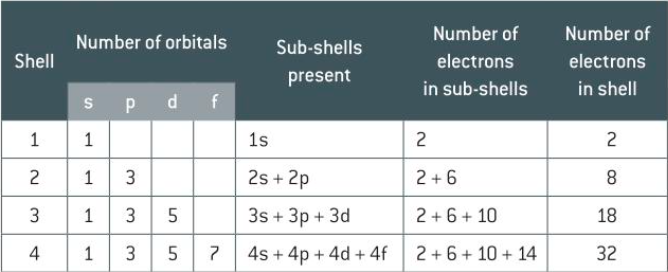
shell and orbital table
each new shell gains a new type of orbital
what’s the difference between orbitals and sub-shells
sub-shells are groupings of orbitals
orbital - the space around a nucleus that has a chance of an electron being there
sub shell - the path taken by electrons as they move
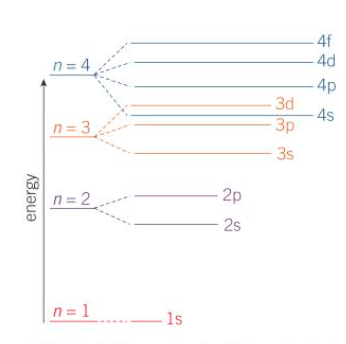
how are orbitals filled + example
by increasing energy

energy levels of sub shells 1-4
this means the 4s sub shell will fill before the 3d sub shell
what is spin
a property an electron has referring to its momentum (don’t need to know what it is just that electrons have it)
what are the 2 types of spin
up spin and down spin
electrons pairs and spin
electron pairs have to have opposite spin as it counteracts the repulsion between the negative charges
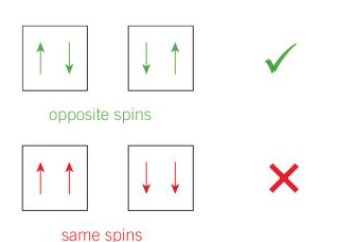
when putting electrons in orbitals how are they placed
putting 1 in each orbital before pairing them

how to draw electron configuration - example
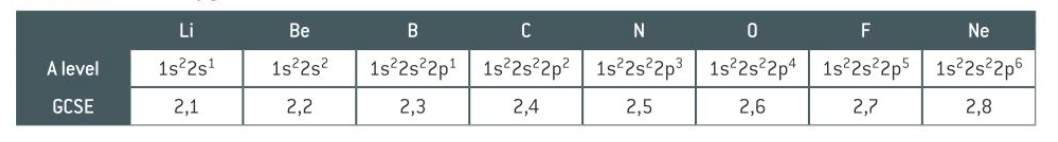
what is shorthand electron configuration
writing the previous noble gas then carrying on the electron configuration so you don’t have to write it all down
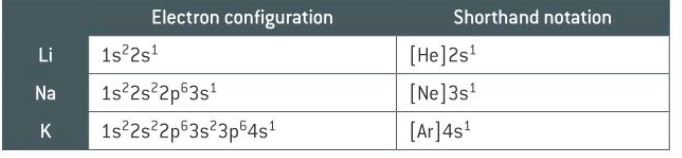
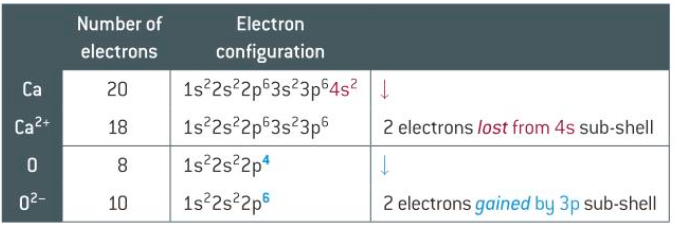
how do you represent ions using electron configuration
you add or remove electrons from the highest energy sub shell
example of calcium and oxygen ions