Week 6 objectives
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What are the characteristics of cancer
Characteristics of cancer:
1. independent of external growth signals (growth on its own)
2. Insensitive to external anti-growth signals (no checks and balances)
3. able to avoid apoptosis (no cell death)
4. capable of indefinite replication (constantly growing)
5. capable of sustained angiogenesis (oxygen and energy) (creates its own blood supply)
6. Capable of tissue invasion and metastasis (spreading and invading in the body)
What is a Genetic variant
Genetic variant = DNA sequence change (mutation/polymorphism)
- May be benign, pathogenic, or of unknown significance
What is a germline variant
Germline variant = DNA variant that occurs in the reproductive cell (egg/sperm) that becomes incorporated into the DNA of every cell in the body of the offspring.
- Variant can be passed from parent to offspring – hereditary
What is a somatic variant
Somatic variant = DNA variant that occurs after conception, not present in the germline (spontaneous/sporadic)
- Can occur in any cells except germ cells
- Variants are NOT passed on to offspring
What are examples of cancer-related oncogenes
Oncogenes = KRAS, HER2/neu,
What are examples of cancer related tumor suppressor genes
Tumor suppressor = TP53, BRCA 1 & 2, RB1
What are the 2 types of tumors and the classifications within each?
Types of tumors:
1. Solid tumors = designated by tissue of origin
- May remain in the original region (benign) or may metastasize (malignant)
Types/classifications: based on tissue of origin
- Carcinomas = epithelial tissue
- Sarcomas = bone, cartilage, muscle, blood vessels, fat
- Teratocarcinomas = multiple cell types
2. Hematological tumors = tumors that arise from WBCs
Types/classification:
- Leukemias = increased WBCs in peripheral blood and bone marrow
- Lymphomas = tend to form masses in lymphatic system
What are some DNA mutations that lead to cancer? Proto-oncogenes specifically
Mutations in DNA that lead to cancer:
- Point mutations, deletions, insertions, tandem duplications, gene amplification (CNV), translocations
mutational mechanisms that lead to proto-oncogenes…
single point mutations that alter protein function, translocations that increase the expression of an oncogene, chromosome translocation that produces a novel product with oncogenic properties, & gene amplification leading to excessive amounts of gene product
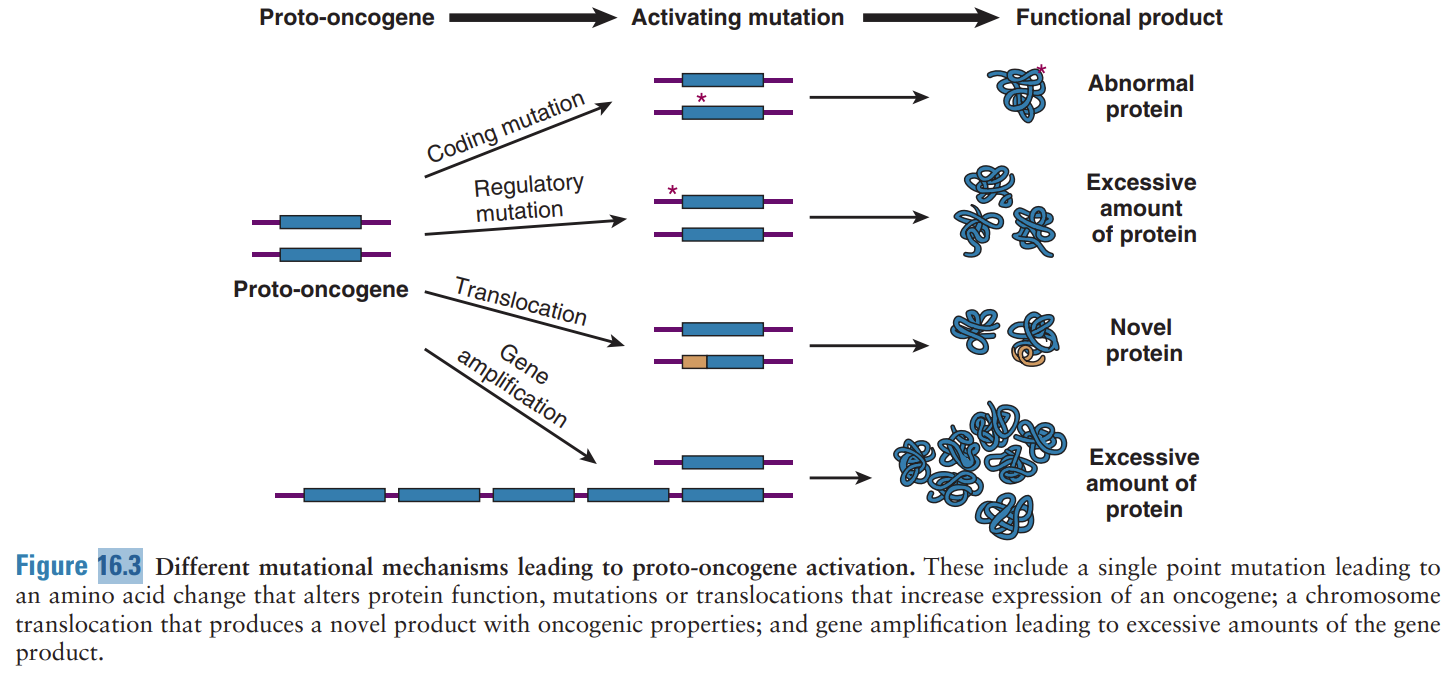
what is a proto-oncogene
Proto-oncogenes = promote cell division and support cell survival (NORMAL VERSION OF ONCOGENES)
- Typically control of cell division and do not allow overgrowth
- When mutated, they become cancer promoting genes (oncogenes)
o Mutations in oncogenes = gain of function mutations
§ Includes gene amplification and upregulation
§ Ex: KRAS, HER2/neu
- Ex: cell membrane receptors for growth factors, hormones & genes that inhibit apoptosis (self-directed cell death)
- Gain of function mutations = amplifications or translocation of DNA regions containing the oncogene or activating mutations
o Causes increased or faulty activity of the proteins
What is a tumor suppressor gene?
Tumor suppressors = slow down or stop cell division (are the brakes)
- Include factors that control transcription or translation of genes required for cell division
- Suppressors also include those proteins that repair DNA damage and promote apoptosis
o Checkpoint proteins suspend cell division when a problem is encountered during DNA synthesis or division
- In cancer, uncontrolled proliferation occurs because of loss of negative regulation, “loss of function” mutations
- Ex: TP53 & BRCA 1/2
- Loss of function mutation = inactivation of suppressor proteins by deletion, translocation, or other mutation
Identify checkpoints in the cell division cycle that are critical for regulated cell proliferation
G1 check point = G1 to S phase
G2 check point = G2 to M phase
*Both checkpoints are usually associated with tumor suppressor genes
What can molecular testing do in oncology
Molecular testing can...
- Determine those patients who are at increased risk of cancer
- Determine tumor characteristics for: Diagnosis, prognosis, and treatment
What is a germline cancer
Germline = hereditary cancers
- Hereditary cancer syndromes result from GERMLINE mutations that can be passed down the family line
o An inherited cancer mutation confers higher risk of cancer throughout the lifetime
§ Doesn't mean you’ll have cancer. Just that you’re at a higher risk
What is germline testing? What does it look at/do
Testing:
- Test for RISK not diagnosis
o Genetic Screening test = help in risk assessment
§ because not diagnosing, just want to see if mutation is present
o A simple peripheral blood sample is usually all that is needed (EDTA purple top)
- Hereditary cancer mutations confer RISK of cancer and NOT a diagnosis of cancer
- Hereditary cancer testing using molecular methods is an opportunity to prevent disease or at least catch it early
- Once level of risk is determined, more aggressive cancer screening can be done (more frequent mammograms, MRI, or ultrasound)
o Occasionally patients with a very high risk may choose to undergo prophylactic surgeries (thyroid removal or mastectomy)
What is a somatic cancer? Testing?
Somatic = sporadic cancers
- Sporadic cancer occurs in somatic cells which is spontaneous and isolated to a particular tissue (until metastasis occurs)
o Sporadic cancers are NOT heritable because the germline is not affected
- Somatic mutations typically ONLY OCCUR IN THE TUMOR
o Just at the tissue type where the cancer arose (mutation only exists in tissue of tumor origin)
o Other cells in the body do not carry these mutations
o Mutations occur in tumor suppressor genes or oncogenes
- Somatic tumor mutation testing is done after the tumor is excised by surgery or on biopsy specimens
o Tumor is formalin fixed and paraffin imbedded
o Laboratory will slice into thin section and place onto slides for further analysis
o Tumor cells can be identified by a pathologist and then extracted by a molecular technologist for mutation testing
- Blood sample or check swab
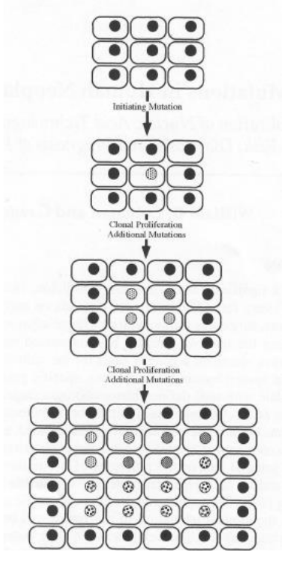
What is tumor heterogeneity
Tumor heterogeneity =
- Alterations in cellular DNA accumulate over time in succeeding generations of daughter cells (clonal expansion)
- Daughter cells with several mutations replace the cells previously comprising the tumor, thus, tumors have high heterogeneity
- Different mutations
What is the two hit hypothesis
Two-hit hypothesis = because cancer is typically a culmination of many things (environmental and genetic risks), there are “2” mutations that lead to cancer
- First hit = usually genetic risk factors
o A recessive inherited genetic variant that provides the predisposition to cancer – but one “good” copy of the gene is still there
o Can be a germline mutation or a heritable mutation
- Second hit = usually a somatic mutation – a mutation in the once “good” version of the gene
o Loss of heterozygosity
o Can also be a detrimental mutation in another gene that works in concert with the “First” hit
- End result = uncontrolled cell division (cancer)
What is retinoblastoma? What’s its role in the 2 hit hypothesis
Retinoblastoma = a rare cancer that results from germline or somatic mutations (or both)
- Example of 2 hit hypothesis
- Mutations occur in RB1 gene (tumor suppressor)
o Loss of function = reduced expression of normal retinoblastoma protein occurs
- First mutated allele is sometimes due to a novel germline mutation
o There is often no family history, but the initial susceptibility to this cancer is in the germline
o First hit can be germline or heritable mutation
- Second mutated allele occurs by somatic mutation
o Either a new mutation or loss of heterozygosity by deletion or hypermethylation (gene silencing)
o Second hit is usually a somatic mutation
What is a bilateral retinoblastoma
Bilateral retinoblastoma = both eyes affected
- Usually present within the first year of life
- Nearly all patients with bilateral disease have germline RB1 mutations
- BUT all patients with germline RB1 mutations do NOT develop disease (still need 2nd hit)
What is a unilateral retinoblastoma
Unilateral retinoblastoma = one eye affected
- Present between 24-30 months of age (older children)
- Due to somatic mutations only, both alleles have to be spontaneously mutated in same cell, making a clonal tumor
- Patients tend to be older because both alleles have to be somatically mutated instead of just one (takes a little longer in life to occur)
What is loss of heterozygosity
Loss of heterozygosity = loss of protective copy of gene, leaving only pathogenic version (allele) behind
- No longer a heterozygote
What is the diagnostic molecular marker
Diagnostic = establishes that a disease is present in the patient (what type of cancer you are looking/working at)
- Ex: presence of Philadelphia chromosome in CML
- What type of cancer? Characteristics?
What is the prognostic molecular marker
Prognostic = association with clinical outcomes such as survival or recurrence-free survival (INDEPDENT OF TREATMENT)
- Just gives prognosis (chances of survival for the given cancer, without considering treatment)
- Ex: P53 mutation indicates more aggressive cancer & HER2/neu overexpression indicates growth and metastasis of breast cancer
- How aggressive? Goals for progression free survival/overall survival?
What is the predictive molecular marker
Predictive = predict activity of a certain therapy response
- Ex: HER2/neu negative tumors do not respond to Herceptin (Trastuzamab)
- Which drug will tumor respond to? Which drugs should be avoided because of predicted resistance?
What is the companion diagnostic molecular marker
Companion diagnostic = type of predictive marker that infers response to particular drug
- Positive test indicates drug may be used (test and drug are co-approved by FDA)
- Ex: BRAF V600E positive melanoma (skin cancer) is sensitive to Vemurafenib and patient must be tested to be eligible for treatment
What is a genetic screening test
Genetic screening test = Assess cancer RISK (likelihood of being diagnosed in the future)
- Disease is NOT present yet, molecular risk analysis provides actionable information for prevention or early detection
- Ex: BRCA testing (predicts risk of primary breast cancer or recurrence)
- Testing germline cellsW
What is a genetic diagnostic test
Genetic Diagnostic test = diagnosis of tumor
- Disease is present, molecular diagnosis establishes its characteristics to provide actionable information for treatment
- Ex: BRAF is a diagnostic test = defined as an aggressive colon/lung tumor with potential for metastasis, as well as resistance to some chemotherapies