2C- Perform Quality Control Procedures
1/27
Earn XP
Description and Tags
TMC: 3 questions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
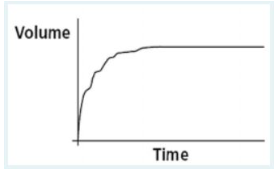
After a patient performs a forced vital capacity (FVC) maneuver, you observe the following volume vs. time plot on the screen of a portable spirometer. What validity error does this plot indicate?
coughing during the maneuver
too slow a start to forced exhalation
breathing during the maneuver
premature end to expiration
coughing during the maneuver
This FVC graph indicates coughing during the maneuver. An abrupt pause occurs about a third of the way through the effort, followed by a short inspiratory effort preceding the cough and then an irregular pattern of exhalation. A normal FVC trace would be smooth throughout.
The package insert quality-control material for a blood gas analyzer defines expected values and upper and lower tolerance levels as follows:
Value Tolerance
pH 7.40 0.5%
PCO2 40 mm Hg 5.0%
PO2 80 mm Hg 3%
Which of the following runs should be closely reviewed?
pH 7.36 PCO2 42 mmHg PO2 79 mmHg
pH 7.44 PCO2 38 mmHg PO2 82 mmHg
pH 7.40 PCO2 40 mmHg PO2 78 mmHg
pH 7.45 PCO2 37 mmHg PO2 81 mmHg
pH 7.45 PCO2 37 mmHg PO2 81 mmHg
The answer that shows a pH of 7.45 and a CO2 of 37 should be examined closely as these values appear to be outside the range of tolerance. If these results are accurate the arterial blood gas machine requires calibration and should not be used to report patient results until maintenance has occurred.
Which of the following factors could negatively affect the results when sampling aqueous quality control material in a blood gas lab?
temperature
tolerance levels
lot number
color
temperature
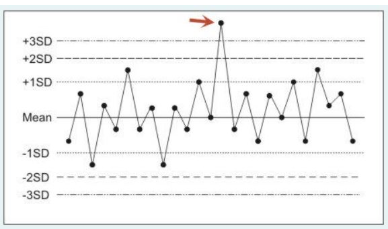
The following figure is a statistical quality control plot for measurements made by a blood gas analyzer. Control run #14 (identified by the arrow) should prompt which of the following actions?
dispose of all control media from the same batch
repair or replace the applicable components
rerun the sample on a different analyzer
send the sample out for proficiency testing
rerun the sample on a different analyzer
Control run #14 represents a random error (imprecision). Typically, random errors are due to statistical probability (a chance occurrence), sample contamination or sample mishandling. When you encounter what appears to be a random error you should either rerun the control or repeat the analysis on a different instrument. In general, one considers repairing or replacing applicable components only when encountering systematic/bias errors. Patient samples are not sent out for proficiency testing. Proficiency testing media (with unknown values) are provided by external labs to assess internal operating procedures and staff competence.
You are checking an ICU ventilator and observe that the exhaled volume is significantly less than the set volume. To determine if this problem is due to an inaccurate ventilator volume control, you should measure the volume at the:
exhalation valve
machine outlet
patient connector
water collection bottle
machine outlet
If exhaled volumes are less than those set on the ventilator, the most likely problems are either 1) system leaks or 2) an inaccurate volume control. Low volumes measured at the exhalation valve or patient connector could be due to either problem. To determine if low exhaled volumes are caused by an inaccurate volume control, you should measure the volume at the machine outlet.
When preparing to use a microprocessor-based mechanical ventilator between patient applications, you should:
change the ventilator circuit and perform a power-on-self-test only
change the circuit and perform a leak test only
change the circuit, perform a leak test and verify the function of alarms only
change the ventilator circuit and perform both a power-on and extended self test
change the ventilator circuit and perform both a power-on and extended self test
You always should confirm a successful power-on self test (POST} before applying a ventilator to a patient and whenever you change the circuit. You should also perform an extended self-test (EST) between each application of a ventilator on different patients.
Which of the following blood gas/hemoximetry quality control procedures is designed to avoid analysis errors associated with deterioration or failure of key analyzer components (filters, membranes, electrodes, etc.)?
performance validation
statistical quality control
preventive maintenance
control media verification
preventive maintenance
Many components used in blood gas analyzers (filters, membranes, electrolyte solution, etc.) have a limited life and deteriorate or fail over time. This results in faulty analysis. Regular preventive maintenance is the best way to avoid these problems. Preventive maintenance should include both scheduled parts replacement and routine function tests, as recommended by the manufacturer.
When reviewing statistical quality control data on a blood gas analyzer, you note a single PCO2 measurement among 30 that falls below the ± 2 SD "in control" standard for your lab. Which of the following is the most likely cause of this error?
contamination of the sample
failure of the PCO2 electrode
incorrect calibrating gas %
incorrect analysis procedures
contamination of the sample
A single pH measurement among 30 that falls outside a lab's ± 2 SD "in control" standard represents a random error or error of imprecision. Random errors in blood gas quality control usually are due to statistical probability (a chance occurrence), sample contamination or sample mishandling. Contaminated buffers, incorrect analysis procedures, component failure and incorrect calibrating gas concentrations are causes of systematic or bias errors.
According to ATS recommendations, diagnostic spirometers should be calibrated to within:
± 5% or 100 mL, whichever is greater
± 10% or 500 mL, whichever is greater
± 1% or 10 mL, whichever is greater
± 3% or 50 mL, whichever is greater
± 3% or 50 mL, whichever is greater
Which of the following blood gas sampling errors would tend to result in a falsely low PCO2?
air contamination of sample
admixture with venous blood
collection in plastic syringe
delay in sample analysis
air contamination of sample
Preanalytic arterial blood gas sampling errors that would tend to result in a falsely low PCO2 include air contamination and dilution with excess heparin. A delay in analysis (metabolic effects) or collection or mixing with venous blood would falsely raise, not lower the PCO2. Use of plastic syringes can cause errors in PO2 measurement (due to gaseous diffusion through the syringe wall), but generally not the PCO2 (being a larger molecule and thus less diffusible through plastic).
The therapist is observing the quality control results in order to determine the accuracy and precision of the blood gas analyzer. According to the documentation that came with the quality control material, pH is supposed to analyze at 7.40 with 0.5% upper and lower standard deviation. The PCO2 analyzer is supposed to measure 30 mmHg with 5% upper and lower standard deviation tolerance. Which of the following runs are consistent with an analyzer that is out-of-control?
pH PCO2 (mm Hg)
Run 1 7.37 25
Run 2 7.41 31
Run 3 7.34 29
2 only
3 only
1 and 3
1 only
1 and 3
How would the respiratory therapist assure that a bedside spirometer is accurate prior to testing a patient?
Perform a pre-operational test.
Test the patient on two different spirometers and assure correlation.
Review the most recent PM report.
Test with a 3-liter super syringe.
Test with a 3-liter super syringe.
When performing a leak test on a volume ventilator, you should:
set the tidal volume at 2 L
set the flow control at maximum
open the breathing circuit to the atmosphere
set the high pressure limit at maximum
set the high pressure limit at maximum
When performing a leak test on a volume ventilator, you should set the high pressure limit at maximum. The system is leak-free only if the pressure 'maxes out' when the machine is triggered against a closed/occluded circuit.
The following patient data is observed:
Blood gas and CO-oximeter results
pH 7.35
PaCO2 27 torr
PaO2 40 torr
Hemoglobin 14.0 g/dL
Oxyhemoglobin 96%
HCO3- 13 mEq/L
Carboxyhemoglobin 2%
Methemoglobin 2%
SaO2 76%
BE -11 mEq/L
The respiratory therapist should:
perform a calibration of the instrument and repeat the analysis
report SaO2 as 76%
administer sodium bicarbonate
perform a calibration of the instrument and repeat the analysis
How often should you conduct blood gas or hemoximetry calibration verification using control media?
at least three control should be analyzed every 8 hours
at least one control should be analyzed every hour
at least one control should be analyzed every 24 hours
at least one control should be analyzed every 8 hours
at least one control should be analyzed every 8 hours
Calibration verification establishes and periodically confirms the validity of blood gas analyzer results. As a general recommendation, at least one control should be analyzed every 8-hour shift. A rotation system should assure that all three levels of the control media are analyzed at least once each 24 hours.
When reviewing blood gas statistical quality control data, you note a progressive positive bias error in the instrument’s PO2 measurement. Which of the following is the most likely cause of this error?
mishandling of the sample
incorrect calibrating gas %
statistical probability error
contamination of the sample
incorrect calibrating gas %
Systematic or bias errors that appear in blood gas statistical quality control analysis are usually due to contaminated buffers, incorrect calibrating gas concentrations, incorrect analysis procedures, or component failure. Sample mishandling, sample contamination and chance statistical or probability errors are causes of imprecision, not bias.
The patient results from a Co-oximeter are questionable and do not fit the clinical scenario presented. In order to assess whether the machine is accurate, the respiratory therapist should
compare the results with that of a pulse oximeter
calibrate the instrument and repeat the patient sample
run quality controls and evaluate results
perform proficiency testing
compare the results with that of a pulse oximeter
The respiratory therapist should always compare laboratory results with the clinical picture. In the case of a mismatch, or questionable result, one should utilize quality control measures to assess accuracy and calibration methods to make adjustments when instruments are inaccurate.
Which of the following is an appropriate plan for regular calibration of a portable bedside spirometer?
perform volume calibration daily and flow calibration weekly
perform both volume calibration and flow calibration daily
perform both volume calibration and flow calibration monthly
perform volume calibration weekly and flow calibration daily
perform volume calibration daily and flow calibration weekly
Portable spirometers require regular calibration to ensure accuracy. Most incorporate computerized calibration checks that prompt you through the steps in this procedure. Calibration involves comparison of the device's volume and flow measurement to a standard. Typically, you perform volume calibration daily and flow calibration weekly.
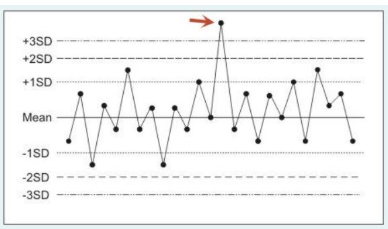
The following figure is a statistical quality control plot for measurements made by a blood gas analyzer. What type of error is represented by control run #14 (identified by the arrow)?
a computation error
a systematic error
a random error
a bias error
a random error
Point A on the plot represents a random error, i.e., a sporadic out of range data point representing instrument imprecision. Typically, random errors are due to statistical probability (a chance occurrence), sample contamination or sample mishandling.
After analyzing arterial blood on a patient with a history of arterial blood gas analysis, the respiratory therapist concludes the results are not possible. They are very different than the patient's historical blood gas results. Quality control records show no evidence of any trends, shifts, or out-of-control situations in the last month. The therapist should
Inform the physician of the suspect blood gas
Ask the medical director for direction in this situation
Perform a two-point calibration on the analyzers
Repeat the analysis with the same blood sample and same analyzer
Repeat the analysis with the same blood sample and same analyzer
The therapist is observing the quality control results in order to determine the accuracy and precision of the blood gas analyzer. According to the documentation that came with the quality control material, pH is supposed to analyze at 7.40 with 0.5% upper and lower standard deviation. The PCO2 analyzer is supposed to measure 30 mmHg with 5% upper and lower standard deviation tolerance. Which of the following runs are consistent with an analyzer that is out-of-control?
pH PCO2 (mm Hg)
Run 1 7.37 25
Run 2 7.41 31
Run 3 7.34 29
1 and 3
2 only
3 only
1 only
1 and 3
The following results are obtained from a calibration study with the slow depression of 3.0-liter super syringe in a pulmonary function lab.
1st calibration 2nd calibration 3rd calibration
2.92 L 2.83 L 2.80 L
What should the respiratory therapist recommend?
Check the system for leaks
Replace the super syringe.
Repeat the calibration using a rapid, consistent depression of the syringe.
Proceed with patient testing.
Repeat the calibration using a rapid, consistent depression of the syringe.
Upon inspection of a FVC graph obtained on an adult patient using a portable spirometer, you observe an 'S' shaped curve. This most likely indicates which of the following?
poor effort at the start of the breath
coughing during the maneuver
prematurely stopping exhalation
breathing during the maneuver
poor effort at the start of the breath
To validate pulse oximetry readings on a critically ill patient, you would compare the saturation reading obtained by the device to which of the following?
the SaO2 measured by a laboratory hemoximeter
the SaO2 reported by a laboratory blood gas analyzer
the PaO2 measured by a transcutaneous monitor
the PaO2 measured by a laboratory blood gas analyzer
the SaO2 measured by a laboratory hemoximeter
Which of the following blood gas sampling errors would tend to result in a falsely low PO2 in a normal patient breathing room air?
use of a plastic syringe
use of a short-bevel needle
delay in sample analysis
air bubbles in the sample
delay in sample analysis
An air/oxygen blender is being used to deliver an FIO2 of 0.60. The best method to assess the accuracy of the FIO2 delivered by the blender would be:
Disconnect the blender and check for alarms
Call engineering to check the blender before use
Calibrate the blender before use
Use an oxygen analyzer in-line
Use an oxygen analyzer in-line
Which is most likely needed to calibrate a 3.0 L syringe, used for evaluating the accuracy of pulmonary function equipment?
a pneumotachometer
a spirograph
a pressure differential device
a respirometer
a respirometer
Which of the following devices would you select to calibrate a body plethysmograph's pressure transducer?
Clark electrode
aneroid manometer
U-tube water column
precision flowmeter
U-tube water column