7.2 Ionisation Energy
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1st Ionisation energy
The energy required to remove 1 mole of electron from 1 mole of a gaseous element
2nd Ionisation energy
The energy required to remove 1 mol of electron from 1 mol of a gaseous ion to form 1 mol of a gaseous 2+ ion
What affects ionisation energy
Atomic radius
Electron shielding
Nuclear attraction
( if outermost electron is easier to lose I.E decreases, if outermost electron is harder to lose I.E increases)
Why is the 2nd Ionisation energy greater than 1st
Nuclear attraction increases
More energy is required to remove another electron
How to identify an element from its successive ionisation energies (period of element is given)
Identify where the biggest difference is (e.g between the 3rd and 4th I.E)
Therefore the group of the element will be the 3rd
Locate the element that lies in the given period within the 3rd group

Explain which element has the higher/lower I.E and why
Boron has a lower I.E because it has an unpaired electron in the 2p sub shell which has a higher energy than electrons in the 2s sub shell. This means that it is easier to remove that electron from Boron than it is from Beryllium
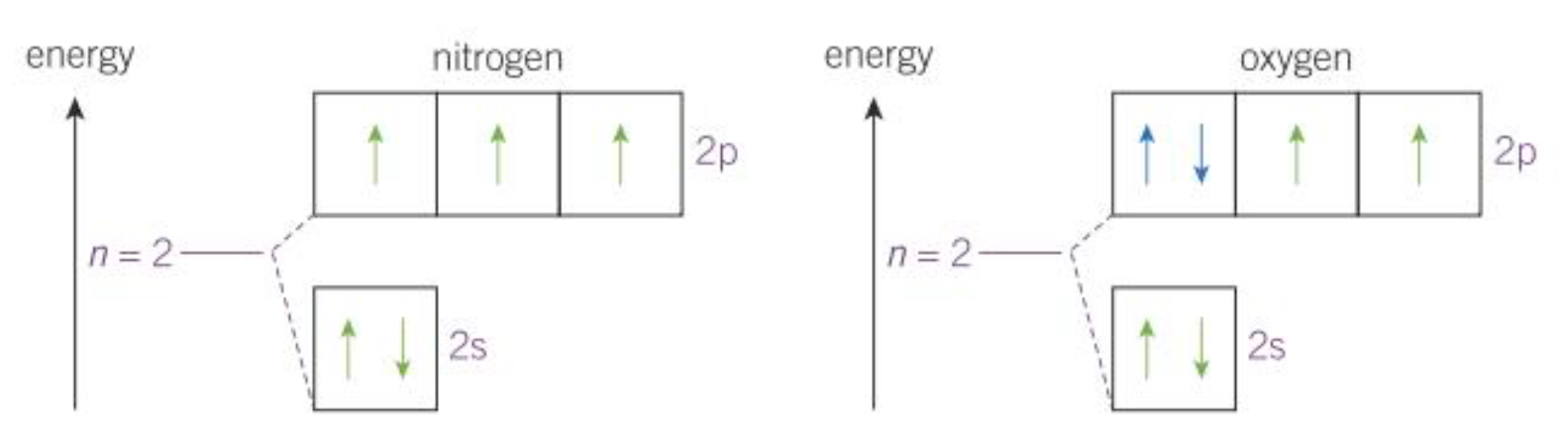
Explain which element has the higher/lower I.E and why
Both Nitrogen and Oxygen have the highest energy electrons in the 2p sub shell
In oxygen there is an orbital with a paired electron that repel, making it easier to an electron in comparison to Nitrogen meaning that Oxygen has a lower I.E