Chem 1C FINAL
0.0(0)
Card Sorting
1/25
There's no tags or description
Looks like no tags are added yet.
Last updated 3:46 AM on 6/12/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
1
New cards
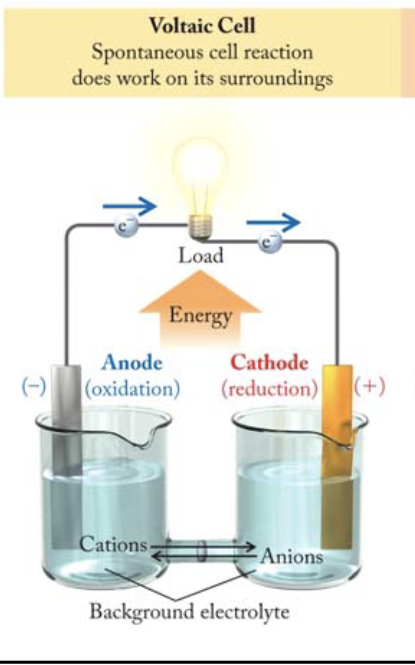
Galvanic (Voltaic) cells (discharge)
use a spontaneous (ΔG < 0) reaction to produce electricity, always from anode to cathode
2
New cards
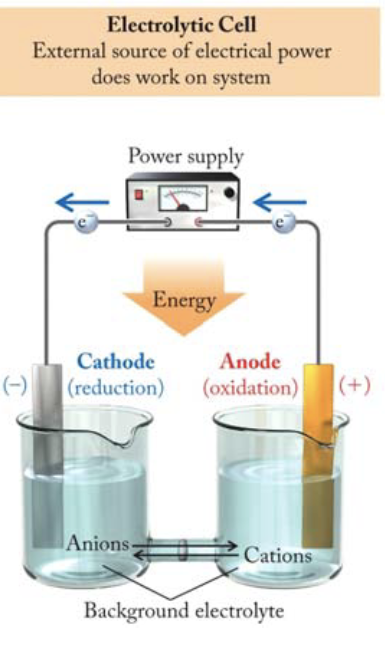
Electrolytic cells (recharge)
use a source of electricity to drive a non‐spontaneous reaction. non-spontaneous meaning ΔG > 0, always from anode to cathode
3
New cards
oxidation
losing electrons, happens at the anode

4
New cards
reduction
gaining electrons, happens at the cathode

5
New cards
Salt bridge
a bridge connecting the two solutions of the cell; balances the flow of electrons, eliminates the accumulation of charge
6
New cards
anode (3 points)
The electrode where electrons are produced, negative, where anions migrate toward
7
New cards
cathode
The electrode where electrons are consumed, positive, what cations migrate toward
8
New cards
double vertical lines represents ___, one vertical lines represents___
a bridge, a phase change
9
New cards
For a cell diagram you should include
concentrations and partial pressures of any gasses if known
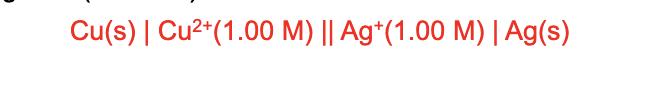
10
New cards
Electromotive Force (emf)
The force pushing electrons through an electrical circuit; also called voltage
11
New cards
Cell Potential (Ecell)
The emf of an electrochemical cell
12
New cards
Standard Cell Potential (E **NOD** cell)
The more positive the potential is the more likely it will be reduced
13
New cards
E **NOD** SHE
0\.00V, can serve as an anode or cathode
14
New cards
positive E **NOD** cell means
a working battery
15
New cards
negative E **NOD** cell means
a dead battery
16
New cards
E **NOD** cell < 0
favors the reactants
17
New cards
E **NOD** cell > 0
favors the products
18
New cards
ΔG < 0
favors the products
19
New cards
As reduction potential increases, the ease of reduction___
increases
20
New cards
a substance with a higher reduction potential will…
oxidize a substance with a lower reduction potential
21
New cards
__ __and__ __ are strong reducing agents
sodium and potassium
22
New cards
Reduction potential is based on
concentration and pH
23
New cards
The higher reduction potential(V), the ___ reducing agent, the ___ oxidizing agent
weaker, stronger
24
New cards
As cell potential increases, the amount of work available ___
increases cause W= -C x Ecell
25
New cards
the amount of work done by an electrochemical cell depends on
the amount of charge transferred
26
New cards
Metal with a lower reduction potential can be used as a sacrificial metal to protect another \n metal with a ____ reduction potential.
higher