3.1.2.4-5 - Empirical and molecular formula, equations and calculations
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Empirical formula definition
Smallest whole number ratio of atoms of each element in a compound
Example empirical formula calculation
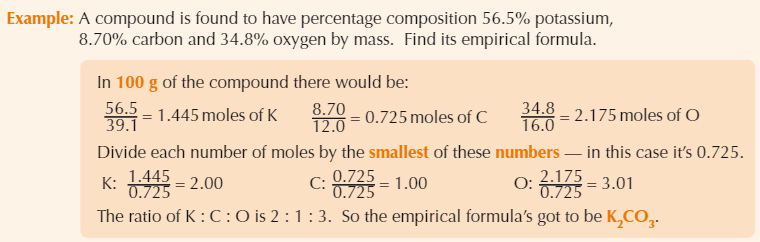
Molecular formula
Gives the actual numbers of atoms of each element in a compound
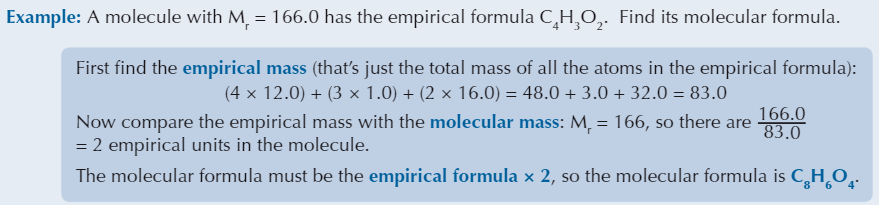
Relationship between molecular and empirical formula
Molecular formula is made up of a whole number of empirical units
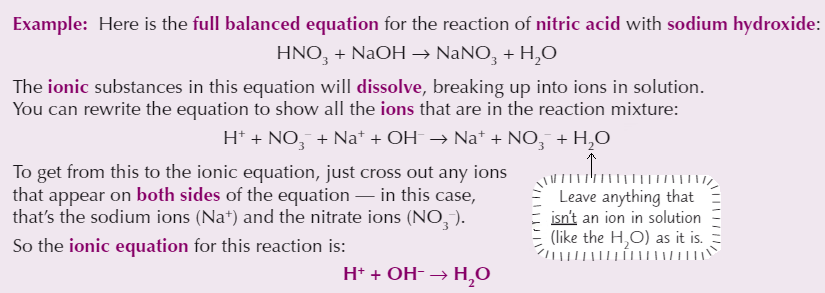
Ionic equation
Can be written for any reaction involving ions that happens in solution
Only includes reacting particles (and the products they form)
Charges must be balanced on either side
Spectator ion
Ion that’s present in reaction mixture, but not involved in reaction
State symbols
s = solid
l = liquid
g = gas
aq = aqueous (solution in water)

Theoretical yield
Mass of product that should be formed in reaction - assuming no chemicals are lost in process
Calculated using masses of reactants and balanced equation
Actual yield is always ____ than theoretical yield
less - because not all chemicals react fully, or another reason
Formula for percentage yield
Percentage yield = Actual yield / Theoretical yield * 100
Atom economy
A measure of the proportion of reactant atoms that become part of desired product (rather than by-products)
Formula for atom economy
% atom economy = Mᵣ of desired product / sum of Mᵣ of all reactants * 100
Example atom economy calculation
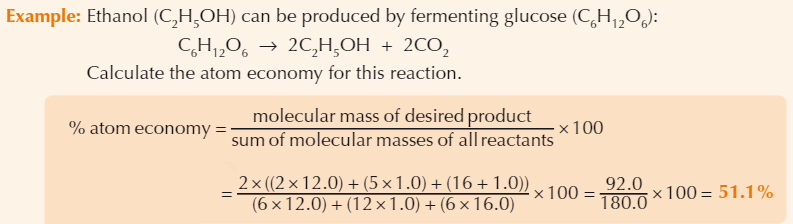
Advantages of processes with high atom economies
Better for environment - less waste
More sustainable - make more efficient use of raw materials
Less expensive - less money spent on separating waste from desired product