Atoms, Elements and compounds - C2
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Isotopes
The same element but different amount of neutrons
Ions
A charged element or compound (An element or compound with less or more electrons than protons)
Nucleon number/atomic mass
The number of nucleons and protons added together
Atomic number
The number of protons
groups
long (colum)
periods
wide (rows)
compound
2 or more different elements that are chemically bonded together
molecules
Two or more elements chemically bonded together
mixture
Two or more different elements or compounds not bonded together (easily separable)
Hydroxide (formula)
OH-
Sulphate (formula)
SO42-
Nitrate (formula)
NO3-
Carbonate (formula)
CO32-
Ammonium (formula)
NH4+
ionic bond
bond between a cation(positive) and anion(negative)
cation
positive charged ion
anion
negativly charged ion
covalent bond
The bond where to non-metals share 1 or more electrons
intramolecular
The force between atoms within a molecule (h2o the force between oxygen and the hydrogen)
intermolecular
The force between different molecules (like h2o and another h2o)
lattice structure
A regular repeating pattern, they are usually found in ionic compounds and covalent compounds
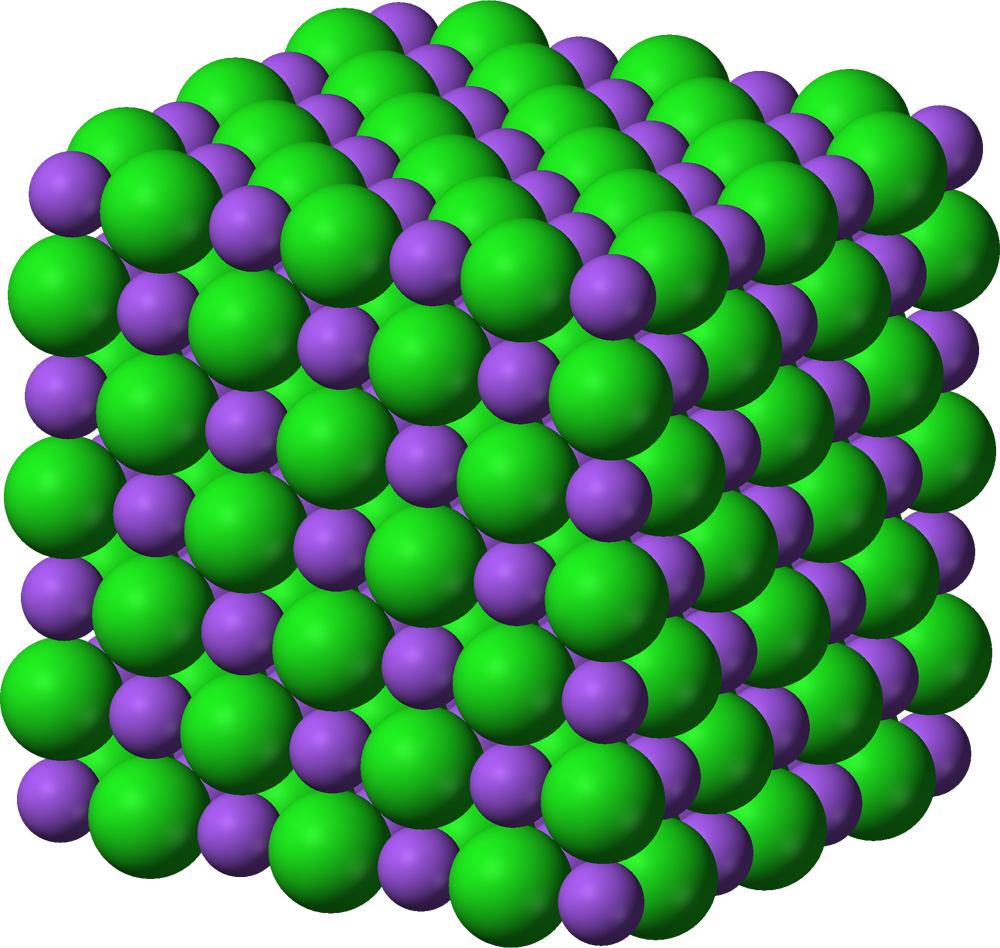
macro molecules
A structure made up of many atoms all covalently bonded to each other
Metallic bonding
A bond between the positively metal ions and their delocalised electrons
malluable
bendable and able to be reshaped
Alloys
A metal mixed with one or more different elements
valence electrons
The electrons in the outer most shell in an atom.
Group uno
The Alkali Metals
Group ocho
The Noble Gases
Group siete
The Halogens
No group elements(between group 2 and 3)
The Transition metals
(PO4)3−
Phosphate