Lecture 20 - bioenergetics
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
what do you use to deliver large amounts of energy into synthesis pathways?
(usually using NADPH
Why Not Just Use Electron Carriers (like NADH) all the time?
Electron carriers store large amounts of energy from oxidation of reduced carbon compounds.
But they are not ideal for quick, small, versatile energy use in diverse reactions.
ATP likely evolved early in evolution to serve as a universal, adaptable energy currency for cells.
Energy from macronutrients (carbs, lipids, amino acids) can be captured as what?
form of ATP.
the cell’s “energy currency”
what processes does atp link together?
Catabolism (breakdown → energy release)
Anabolism (biosynthesis → energy required)
ATP is formed during catabolic reactions and used during anabolic processes.
ATP levels stay relatively constant—its breakdown is matched by its synthesis.
what is happening to atp during anabolism and catabolism?
ATP → ADP + Pi during anabolism (energy-consuming work).
ADP + Pi → ATP during catabolism (energy-releasing breakdown).
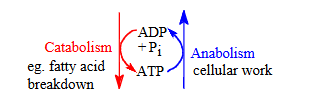
so what does ATP serve as?
ATP serves as a major linking intermediate between energy yielding and energy requiring reactions. [ATP] normally remains relatively constant (in a steady state) whereby ATP breakdown = ATP formation
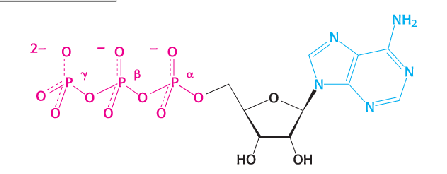
what is this structure?
ATP
Adenosine triphosphate
Reasons why ATP is the “energy currency” in cells include? (4)
Yields free energy from hydrolysis
Intermediate phosphoryl group transfer potential
Stability
Versatility, i.e. it can function as an energy “currency”
what does hydrolysis of ATP leads to?
ATP can be broken down (hydrolyzed) into:
ADP + Pi
AMP + PPi
etc.
Each of these reactions has a large negative ∆G°′, meaning they release free energy and are spontaneous under standard conditions.
Because of this, ATP hydrolysis can be coupled to endergonic (non-spontaneous) reactions to drive them forward—this is how ATP does work in the cell.
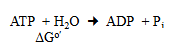
how much energy does this reaction release and state whether it is exer or ender
−30.5 kJ/mo
exergonic
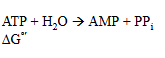
do the same with this one
-45.6 kJ/mol
exergonic
does PPi (inorganic pyrophosphate) contain energy?
yes it contains energy aswell
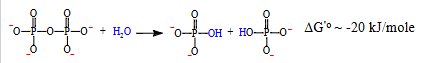
how can endergonic reactions use atp for their advantage?
Reactions that are highly endergonic (require a lot of energy) often use ATP → AMP + PPᵢ and then hydrolyze PPᵢ to make the reaction strongly favorable.
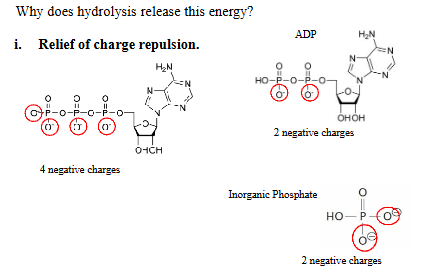
Why Does ATP Hydrolysis Release So Much Energy?
ATP has 4 negative charges closely packed on the phosphate groups.
Upon hydrolysis:
ADP has 2 negative charges
Inorganic phosphate (Pᵢ) also has 2 negative charges
This spreads out the negative charges, relieving electrostatic repulsion, which stabilizes the products and makes the reaction more exergonic (energy-releasing).
Picture showing why
additional factors why atp releases so much energy? (3)
More Resonance Forms in Products
Proton Dissociation
Hydration (Solvation)
explain why More Resonance Forms in Products release energy from atp
ADP + Pi have more resonance structures than ATP.
This increases entropy and stabilizes the products.
Greater resonance means more delocalization of electrons, which lowers the free energy of the products.
explain why Proton Dissociation releases energy from atp
When hydrolysis occurs, the product ADP immediately dissociates one more proton, a spontaneous reaction that contributes to the overall –ve ∆G for the hydrolysis
explain why Hydration (Solvation) leads to energy release from ATP?
The hydrolysis products from ATP, ADP + Pi, are solvated by water molecules, further stabilizing the products relative to the reactants
why is The phrase "high-energy phosphate bond" is misleading.
used by biochemists to describe the P-O bond broken in
hydrolysis reactions, is incorrect and misleading as it
wrongly suggests that the bond itself contains the
energy
so what the proper terminoly?
Breaking bonds requires energy, but ATP hydrolysis is favorable because:
The products are lower in free energy due to:
Relief of charge repulsion
More resonance forms
Proton dissociation
Hydration stabilization
In fact, the breaking of all chemical bonds requires an input of energy. The free energy released by hydrolysis of phosphate compounds does not come from the specific
bond that is broken; it results from the products of the reaction having a lower free-energy content than the reactants
what is atp able to do with its phosphate group
ATP has intermediate phosphoryl group transfer
potential
what is Phosphoryl Group Transfer Potential (PGTP)?
The ability of a phosphorylated compound to give up its phosphate group is known as Phosphoryl Group Transfer Potential (PGTP)
how do you measure PGTP?
The magnitude of this capability under standard conditions is the same as the ΔG°' of hydrolysis for the group, but of opposite sign.
For example, the ΔG°' for ATP hydrolysis is ‒ 30.5 kJ/mol, therefore the PGTP of this phosphate is 30.5 kJ/mol.
Standard Free Energy of Hydrolysis of Some Phosphorylated
Compounds and the thioester, Acetyl-CoA
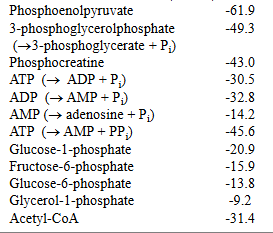
ATP has an intermediate PGTP, meaning:?
It can accept phosphate from molecules with higher PGTP (more negative ΔG°’),
And donate phosphate to molecules with lower PGTP (less negative ΔG°’).
what trait does PGTP give atp?
Versatility
his means that is can easily act as a
phosphoryl group donor (ATP) or acceptor (ADP).
what happens if PGTP is too high or too low
If PGTP were too high:
a) Phosphate transfer would be difficult because few molecules have higher energy.
b) Too much energy would be released—more than needed, wasting energy.
If PGTP were too low:
a) Not enough energy would be released during hydrolysis.
b) Therefore, ATP couldn’t power many energy-requiring processes.
how phosphoenolpyruvate (PEP) can be used to synthesize ATP
Phosphoenolpyruvate has high PGTP so it can transfer its phosphate to ADP in a coupled reaction because the overall coupled reaction has negative ΔG
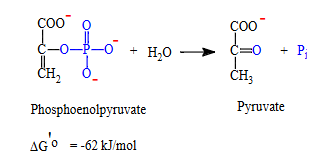
what is reaction of PEP being converted into atp?
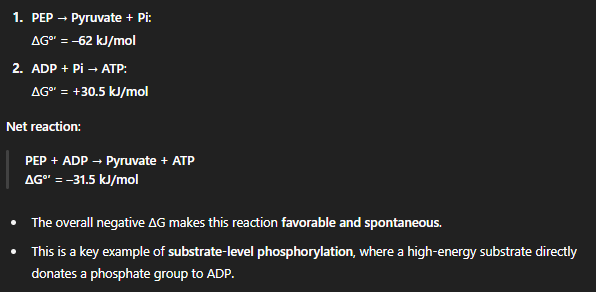
what is Substrate-Level Phosphorylation?
A way to make ATP without using the electron transport chain.
Happens in glycolysis and the citric acid cycle.
Requires a donor like PEP, 1,3-bisphosphoglycerate, or phosphocreatine with higher PGTP than ATP.
how does ATP Helps Drive Endergonic Reactions?

ATP hydrolysis is spontaneous thermodynamically, but it doesn't happen randomly. Why?
High activation energy
Enzyme control
explain why High activation energy prevents ATP hydrolysis from occuring randomly?
ATP hydrolysis requires a lot of energy to get started (220 kJ/mol activation energy).
Without enzymes, ATP is stable and won’t hydrolyze randomly—protecting cellular energy.
why does Enzyme Control affect ATP hydrolysis?
Enzymes specifically regulate when and where ATP is hydrolyzed.
This ensures ATP’s energy is used purposefully for processes like muscle contraction, ion pumping, biosynthesis, etc.
what can happen to the terminal phosphate group of ATP?
The terminal phosphate group of ATP can be transferred (phosphorylation) to many different types of organic molecules including proteins, lipids and sugars.
key enzymes for phosphorylation?
Kinases: transfer phosphate to a molecule.
Phosphatases: remove a phosphate from a molecule.
This makes ATP a flexible and universal regulator in cellular biochemistry.
Why Phosphorylation Is Useful (3)
Increases the free energy of the phosphorylated molecule.
Confers charge to the phosphorylated molecule
Induces protein conformation changes
i) acts as a switch to turn enzymes on and off
ii) pump ions against concentration gradients
iii) turn protein signalling pathways on and off
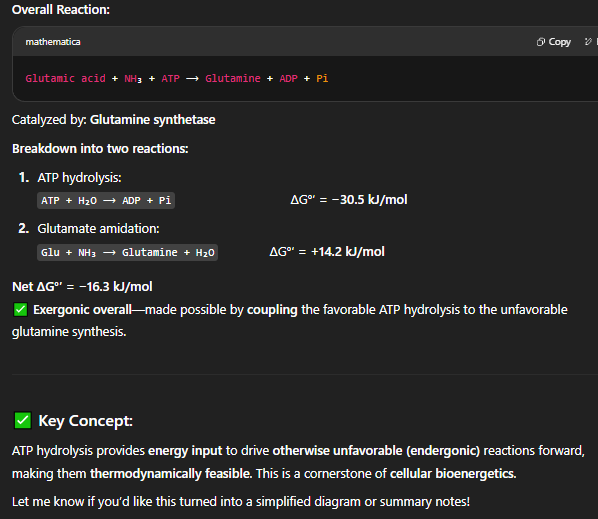
how does Phosphorylation Increases Free Energy?
Direct conversion of glutamate to glutamine is thermodynamically unfavorable.
Instead, the reaction is broken into two steps:
Activate glutamate to glutamate phosphate by phosphorylating it— thermodynamically favourable because it is coupled to ATP hydrolysis
Displacement of glutamate phosphate to glutamine is thermodynamically favourable
🔁 This two-step pathway makes the overall process spontaneous by raising the energy of the intermediate.
the synthesis of glutamine is an example of what?
simple ATP hydrolysis
Glutamine Synthesis via ATP Coupling
how does phosphorylation affect molecule diffusion across membranes?
The addition of a phosphate group (via ATP) adds negative charge, which:
Makes the molecule less permeable through lipid membranes.
Effectively traps it inside the cell because charged molecules can't easily cross the hydrophobic lipid bilayer.
This principle is critical for cellular control of metabolite retention, even if the concentration gradient favors diffusion out of the cell.
what is the first step of glycolysis an example of?
Glucose + ATP → Glucose-6-phosphate + ADP + H⁺
Catalyzed by the enzyme hexokinaseThis phosphorylation:
The first step of glycolysis is an example of phosphorylation providing both an increases in free energy and the trapping of an important metabolite in the cell
📌 This step is irreversible and commits glucose to metabolism inside the cell.
why does Phosphorylation Changes Protein Conformation?
Proteins have a shape defined by their amino acid sequence.
When a phosphate group is added (often to serine), it alters the protein’s structure.
This is because the phosphorylated version is no longer the same protein chemically—it has new electrostatic properties.
how does phosphorylation turn enzymes on or off?
Phosphorylation of an enzyme will frequently cause a conformational change in the protein blocking (off) or opening (on) an active site
what is an ex of phosphorylation turning enzymes on and off?
Pyruvate dehydrogenase (PDH)
Active PDH → Kinase adds phosphate → Inactive PDH
Phosphatase removes phosphate → PDH reactivated
This is reversible and tightly regulated.
Sometimes, phosphorylation activates enzymes; other times, it inactivates them—it depends on the specific enzyme.
how does Phosphorylation Powers Ion Pumps like the sodium potassium pump?
Function: Maintains membrane potential by moving 3 Na⁺ out and 2 K⁺ in.
ATP hydrolysis provides the energy to:
Change the pump's conformation.
Drive ions against their concentration gradients.
Phosphorylation causes the pump to adopt a conformation that favors Na⁺ export.
Dephosphorylation restores the conformation for K⁺ import.
how can Phosphorylation Regulates Signaling Pathways?
Example: Receptor Tyrosine Kinases (RTKs)
A signaling molecule causes receptor dimerization.
This triggers cross-phosphorylation (autophosphorylation) on tyrosine residues.
Phosphorylation:
Activates intracellular signaling cascades
Turns pathways on/off depending on cell needs
what do signaling cascades often involve?
Signaling cascades often involve kinase cascades:
Each level of kinases turns on the level of kinases below it.
Crucial for cell growth, reproduction
and stress responsesWhen these pathways malfunction disease/death result.
ATP is a _____
carrier not a store of chemical energy
how much atp is in the average human?
A human body contains ~0.5 pounds of ATP at any moment.
But it uses ~100 pounds of ATP daily!
how is it possible to use 100 pounds of ATP daily?
This is possible because:
ATP is continuously regenerated from ADP + Pi.
Each molecule of ATP is recycled every ~7 minutes.
This is why ATP is not a store of energy, just a carrier of chemical energy from stores (carbohydrates/fats) to locations/processes that require i
Biologically there are three ways to generate ATP name them?
Substrate level phosphorylation
Oxidative phosphorylation
Photophosphorylation
what is Oxidative phosphorylation
in mitochondria (couples ATP formation to energy-releasing redox reactions)
The synthesis of ATP from ADP and Pi using energy derived from electrons transferred to oxygen via the electron transport chain (ETC) in mitochondria.
what is Photophosphorylation
in chloroplasts (couples ATP formation to energy derived from absorption of visible light: plants and some microorganisms only.
The process of generating ATP from ADP and inorganic phosphate (Pi) using light energy, typically during photosynthesis in plants, algae, and cyanobacteria
what is another type of energy currency?
Another type of energy ‘currency’ is provided by thioester bonds that are not resonance stabilized, and thus store more G than oxygen esters.
The C–S bond in a thioester is less stable than the C–O bond in regular esters.
This makes thioesters more reactive and their hydrolysis releases more free energy.