Orgo CH11/12 Study Guide for Final
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
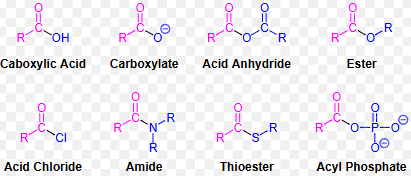
Identify carboxylic acids, acid halides, acid anhydrides, amides, esters, and nitriles, and be able to name carboxylic acids using current IUPAC nomenclature.
Carboxylic Acids: R-COOH; name ends in -oic acid (e.g., ethanoic acid).
Acid Halides: R-COX (X = Cl, Br); name as alkanoyl halide (e.g., ethanoyl chloride).
Anhydrides: R-CO-O-COR; name as alkanoic anhydride (e.g., acetic anhydride).
Esters: R-COOR'; name as alkyl alkanoate (e.g., methyl ethanoate).
Amides: R-CONH₂, R-CONHR’, R-CONR₂; name as alkanamide.
Nitriles: R-C≡N; name as alkanenitrile (e.g., ethanenitrile).
Recognize the general acidity of carboxylic acids in acid-base reactions.
Carboxylic acids are weak acids (pKa ≈ 4–5).
Resonance stabilizes the carboxylate anion, making them more acidic than alcohols.
Determine the product, starting material, and/or reagents for the following reactions to synthesize carboxylic acids:
a. Alkyl benzene oxidation
b. Alcohol and aldehyde oxidation
c. Hydrolysis of carboxylic acid derivatives
a. Alkyl Benzene Oxidation: Use KMnO₄ or H₂CrO₄ to oxidize benzylic carbon to COOH.
b. Alcohol & Aldehyde Oxidation: 1° alcohol or aldehyde + Jones reagent or KMnO₄ → COOH.
c. Hydrolysis of Derivatives: Acid/base hydrolysis of esters, nitriles, amides → COOH.
Determine the product, starting material, and/or reagents for reactions of carboxylic acids to produce acyl chlorides or esters.
To Acyl Chloride: Use SOCl₂, PCl₃, or PCl₅.
To Ester: Use alcohol + acid catalyst (Fischer esterification).
Draw the product, starting material, and/or reagents for the following nucleophilic acyl substitution reactions:
a. Acid Halides conversion to:
ii. Ester
iii. Amide
iv. Alcohol
b. Acid anhydride conversion to:
i. Ester
ii. Amide
iii. Alcohol
c. Ester conversion to:
i. Amide
ii. Alcohol
d. Amide conversion to
i. Amine
a. Acid Halides →
Ester: Add alcohol.
Amide: Add ammonia or amine.
Alcohol: Add water (hydrolysis).
b. Acid Anhydrides →
Ester: Add alcohol.
Amide: Add ammonia or amine.
Alcohol: Add water (hydrolysis).
c. Esters →
Amide: Add ammonia or amine (requires heating).
Alcohol: Add water + acid/base (hydrolysis).
d. Amides →
Amine: Use LiAlH₄ to reduce to amine.
Describe various applications that use carboxylic acid derivative chemistry.
Used in pharmaceuticals (e.g., aspirin = ester).
Important for polymer synthesis (e.g., nylon from diamides).
Widely used in fragrances, soaps, and plasticizers.
Identify a suitable route in a short multistep synthesis of an organic target.
Identify target functional group; work backward (retrosynthesis).
Use known conversions (e.g., alcohol → ester → carboxylic acid).
Minimize steps and choose conditions compatible with all functional groups.
Identify and name amines.
Amines: Nitrogen bonded to alkyl/aryl groups (R-NH₂, R₂NH, R₃N).
Naming:
IUPAC: Identify longest chain, use suffix “-amine” (e.g., ethanamine).
Common: Name alkyl groups alphabetically + “amine” (e.g., dimethylamine)
Describe properties of amines.
Boiling Point: Higher than alkanes, lower than alcohols (H-bonding in 1° & 2°).
Solubility: Small amines are water-soluble due to H-bonding.
Odor: Often fishy or unpleasant smell.
Rank the basicity of different amines.
Rank: 2° > 1° > 3° (in aqueous solution, due to solvation).
Aromatic Amines (e.g., aniline): Less basic due to resonance with benzene ring.
Electron-donating groups ↑ basicity; withdrawing groups ↓ basicity.
Describe spectroscopy techniques to identify and characterize amines.
IR:
1° amine → 2 N-H stretches (~3300 & 3400 cm⁻¹).
2° amine → 1 N-H stretch.
NMR: N-H proton = broad peak (~1-5 ppm); adjacent C-H shifts slightly downfield.
Mass Spec: Characteristic fragmentation near the nitrogen.
Predict the product, starting material and/or reagents for the following reactions to produce amines.
a. Reduction of nitriles
b. Reduction of amides
c. Reduction of azides
d. Reductive amination
e. Reduction of nitrobenzene
a. Reduction of Nitriles
Reagents: LiAlH₄ or H₂/Pd.
Product: 1° amine (R-CN → R-CH₂NH₂).
b. Reduction of Amides
Reagents: LiAlH₄.
Product: 1°, 2°, or 3° amines depending on R-groups.
c. Reduction of Azides
Reagents: H₂/Pd or LiAlH₄.
Product: 1° amine (RN₃ → RNH₂).
d. Reductive Amination
Reagents: Aldehyde/ketone + amine + [H] (NaBH₃CN or H₂/Pd).
Product: New C-N bond (amine).
e. Reduction of Nitrobenzene
Reagents: H₂/Pd or Sn/HCl.
Product: Aniline (Ar-NO₂ → Ar-NH₂).