Percentage Yield + Atom Economy
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Why is it not always possible to obtain the theoretical amount of product in a chemical reaction?
The reaction may be reversible.
Some product may be lost during separation.
Side reactions may occur.
How is the percentage yield of a product in a chemical reaction calculated?
% Yield = (Actual mass of product / Maximum theoretical mass of product) x 100%
What is atom economy?
A measure of the amount of starting materials that end up as useful products. It is the ratio of the relative formula mass of the desired product to the sum of the relative formula masses of all reactants.
What is the % yield of NH₃ if 40.5 g NH₃ is produced from 20.0 mol H₂ and excess N₂? (N₂ + 3H₂ ⟶ 2NH₃)
Step 1: Theoretical moles of NH₃: Ratio H₂:NH₃ is 3:2, so moles NH₃ = 20.0 / 1.5 = 13.3 moles.
Step 2: Theoretical mass of NH₃ = 13.3 x 17 = 227 g
Step 3: % Yield = (40.5 / 227) x 100 = 17.8%.
Look at the equations for the two reactions that produce CuCl2
Reaction I: CuCO₃ + 2HCl ⟶ CuCl₂ + H₂O + CO₂ Reaction II: CuO + 2HCl ⟶ CuCl₂ + H₂O.
(Mr values: CuO = 79.5; HCl = 36.5; CuCl₂ = 134.5; H₂O=18).
Which reaction has a better atom economy?
Reaction II. Total Mr of reactants = 79.5 + (2 x 36.5) = 152.5. Atom Economy = (134.5 / 152.5) x 100% = 88.2%. (Reaction I has a lower atom economy because CO₂ is a waste product).
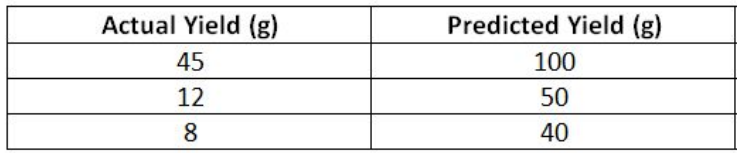
Calculate the percentage yield from the following data.
45
24
20