Chemical Reactions & Types
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
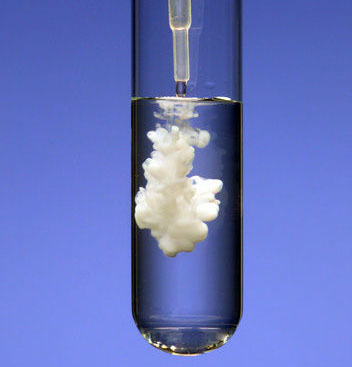
precipitate
Solid compound produced from 2 liquids during a chemical reaction. Sign of a chemical reaction.
bubbles
Gas given off during a chemical reaction; may or may not have an odour. Sign of a chemical reaction.
signs of a chemical reaction
change in temperature, formation of bubbles, precipitate forms, color change, change in smell, difficult to reverse
subscript
Number written after and BELOW an element in a chemical formula telling you how many of the preceding element are needed for that compound or molecule.
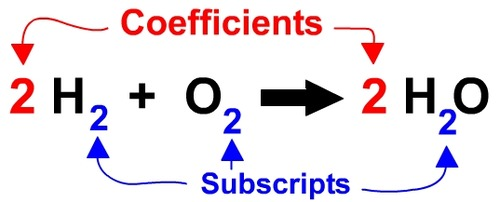
chemical equation
Written representation of a chemical reaction/chemical change; shows all the reactants and products. Always in the form of: Reactants arrow Products.
chemical change
When substances interact with each other. Original substances are used up. Chemical Reaction
physical change
When a substance changes its phase (changes to a solid, liquid , gas, or plasma) or is modified (size, shape, etc) but remains the same substance.

compound
Any amount of a chemical combination of 2 or more different atoms.
molecule
A chemical combination of 2 or more nonmetals held together by covalent bonds
chemical reaction
the process by which one or more substances change to produce one or more different substances
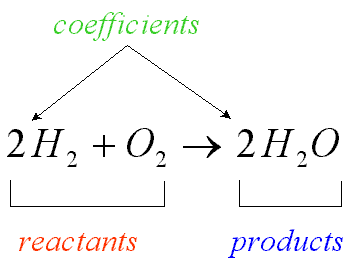
chemical equation
a representation of a chemical reaction that uses symbols to show the relationship between the reactants and the products
reactants
things that react together (on the left side of the equation)
products
things that are produced (on the right side of the equation)
coefficient
numbers written to balance a chemical equation in front of the element or compound
law of conservation of mass
mass is neither created or destroyed, only transformed during reactions, which is why we balance chemical equations
yield
to produce
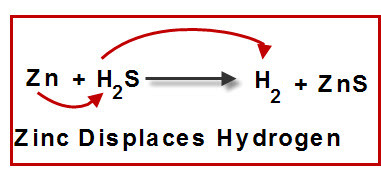
single replacement reaction
a chemical reaction in which one element takes the place of another element in a compound
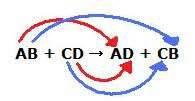
double replacement reaction
a chemical reaction between two compounds where the positive ion of one compound is exchanged with the positive ion of another compound
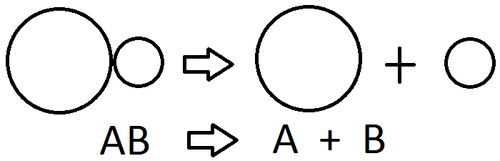
decomposition reaction
a chemical change in which a single compound is broken down into two or more simpler products
synthesis reaction
a chemical reaction in which two or more substances react to yield a single product

combustion reaction
a chemical change in which an organic compound reacts with oxygen to yield CO₂ and H₂O
