Chemistry II Lesson 4: Electronegativity and Hybridization
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
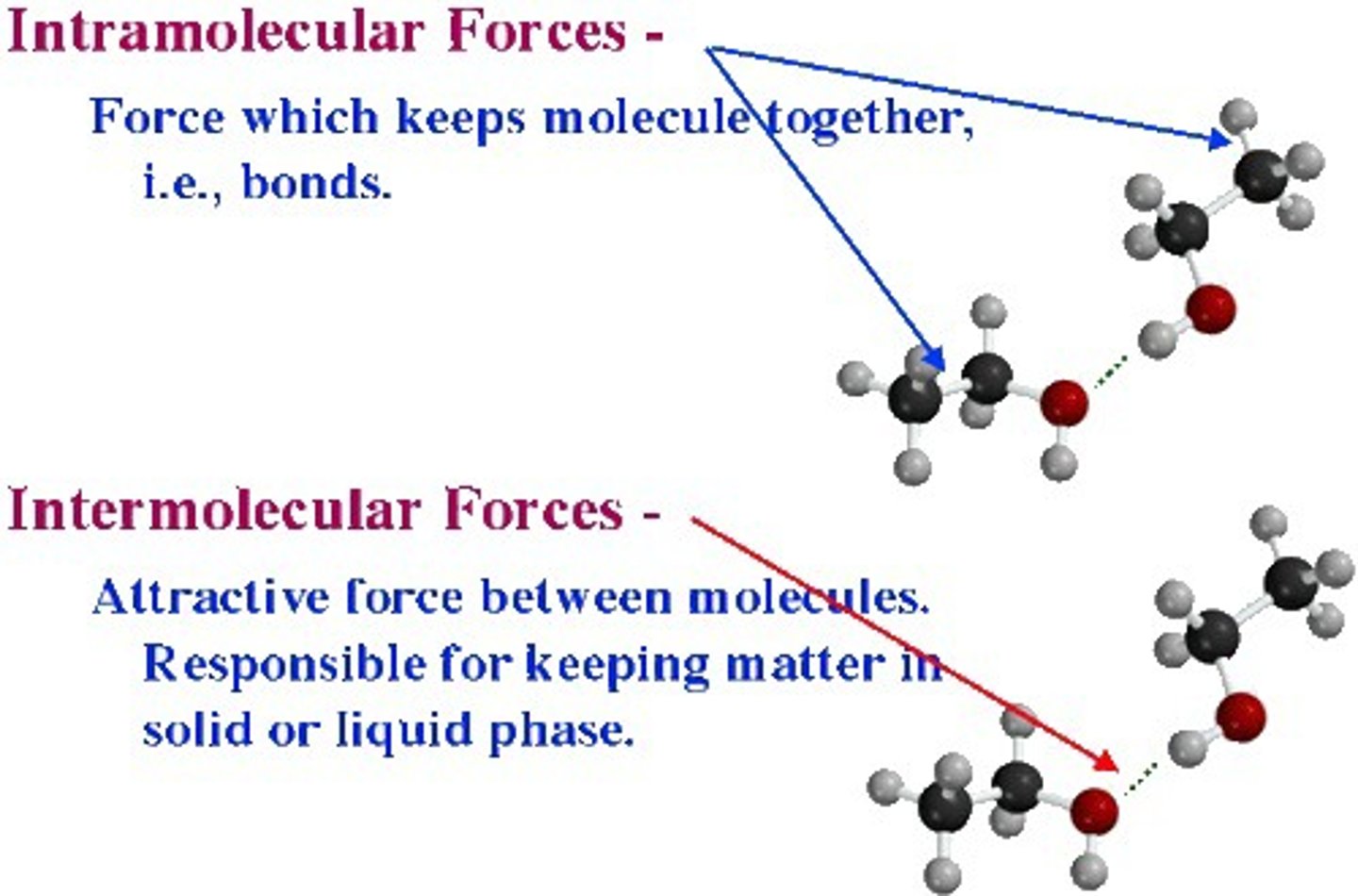
What is the difference between intermolecular forces and intramolecular forces?
Intermolecular Forces are between two difference molecules.
Intramolecular Forces are between the same molecule.
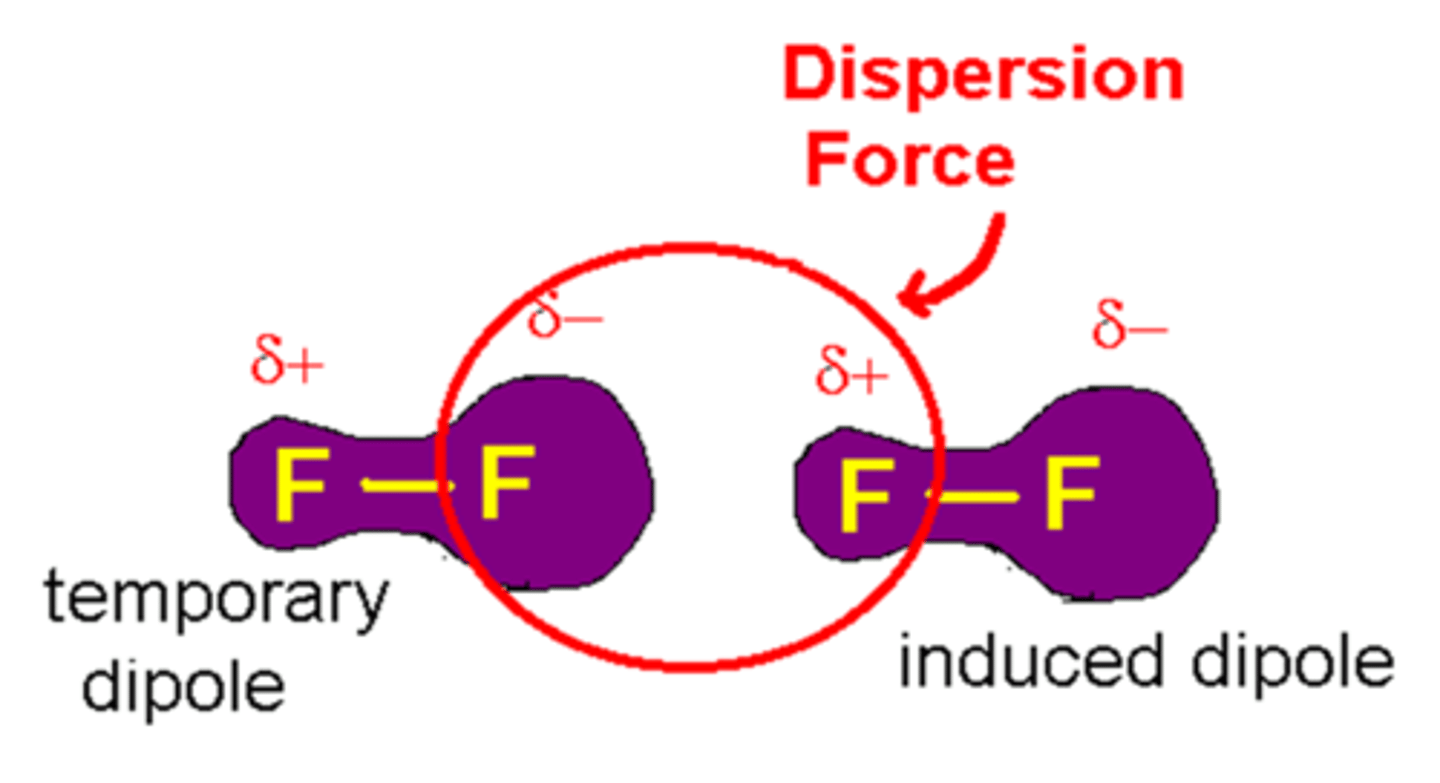
Which intermolecular force is a result of polarization of electron clouds between non-polar molecules?
(A) Dipole-dipole Interaction
(B) Hydrogen Bonding
(C) London Dispersion Forces
(D) Covalent Bonding
(C) London Dispersion Forces
London Dispersion Forces result from the polarization of electron clouds between non-polar molecules.
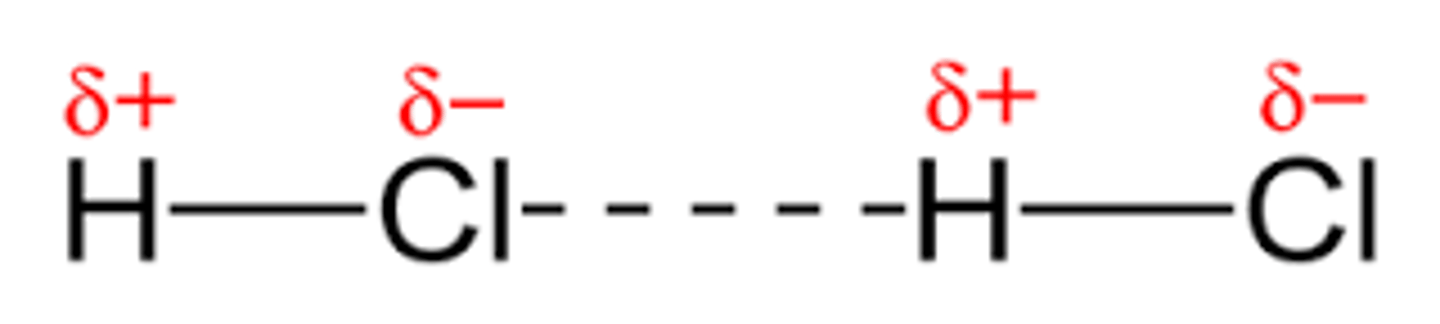
Which intermolecular force is a result of attraction between the oppositely-charged ends of two polar molecules?
(A) Dipole-dipole Interaction
(B) Hydrogen Bonding
(C) London Dispersion Forces
(D) Covalent Bonding
(A) Dipole-dipole Interaction
A Dipole-dipole Interaction is a result of attraction between the oppositely-charged ends of two polar molecules
Put the following bond types in order from weakest to strongest:
I. Dipole-dipole Interaction
II. Hydrogen Bonding
III. London Dispersion Forces
IV. Covalent Bonding
(A) I < III < IV < II
(B) I < III < II < IV
(C) III < II < I < IV
(D) III < I < II < IV
(D) III < I < II < IV
In order from weakest to strongest: London Dispersion Forces < Dipole-dipole Interaction < Hydrogen Bonding < Covalent Bonding

Draw the electron configuration (electron energy levels diagram) for Carbon?
See image.
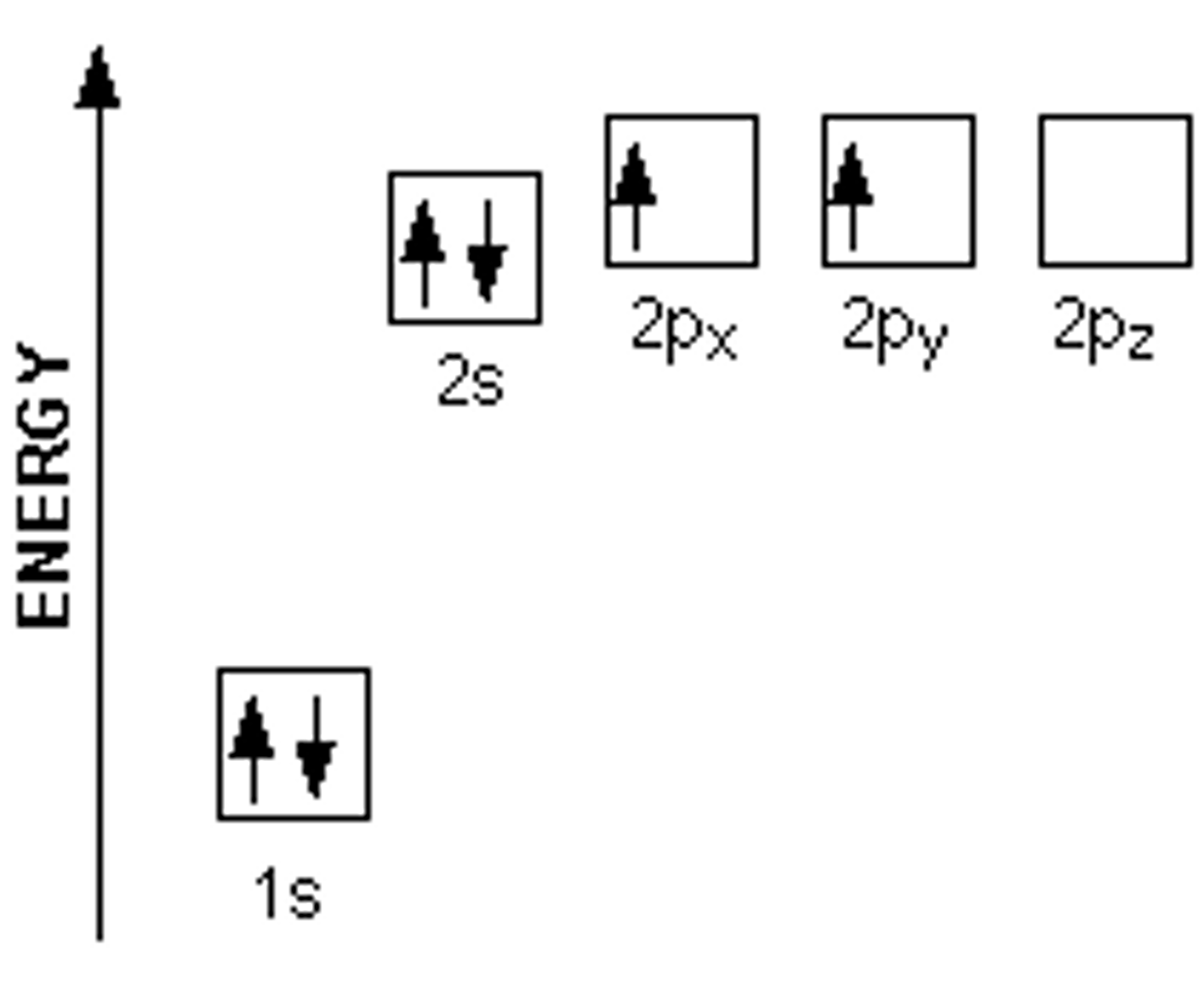
If Carbon only has two 2p electrons, how does it form four bonds to make Methane (CH4)?
One of the 2s orbital electrons jumps up to one of the 2p orbitals. The three 2p orbitals then hybridize with the single 2s orbital, forming four sp3 orbitals with a lone electron in each.
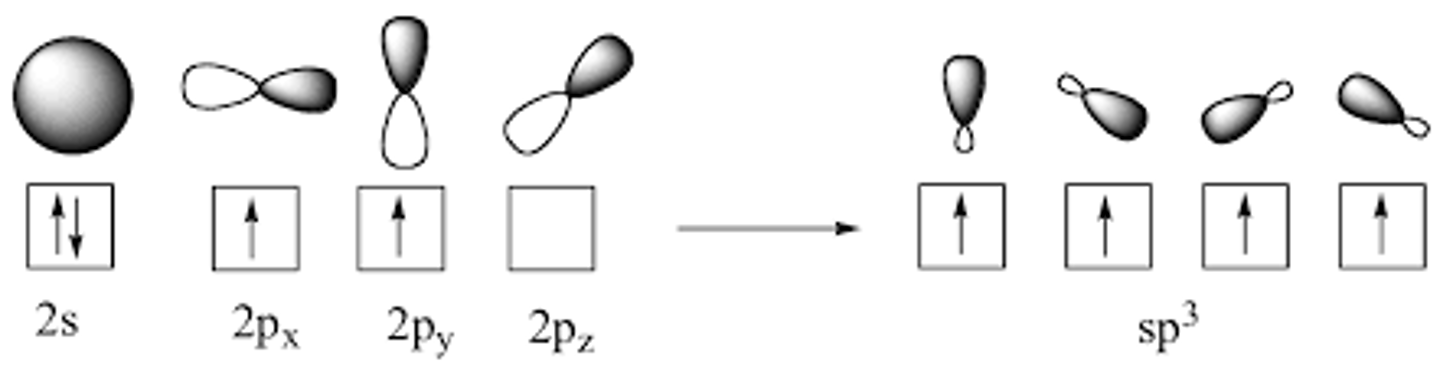
An sp3 orbital has what percent s character and what percent p character?
25 percent s character and 75 percent p character.
Draw the shape of an sp3 orbital.
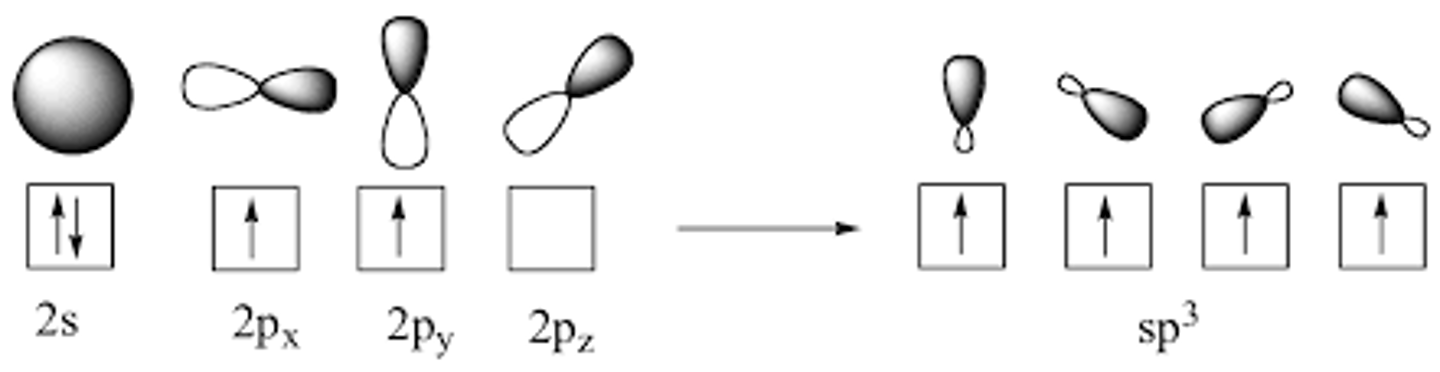
The sp3 orbitals of Carbon join with the s orbitals of Hydrogen to make Methane (CH4), forming what type of bonds?
(A) σ Bond
(B) π Bond
(C) α Bond
(D) β Bond
(A) σ Bond
The sp3 orbitals of Carbon join with the s orbitals of Hydrogen to make Methane (CH4), forming four σ Bonds.
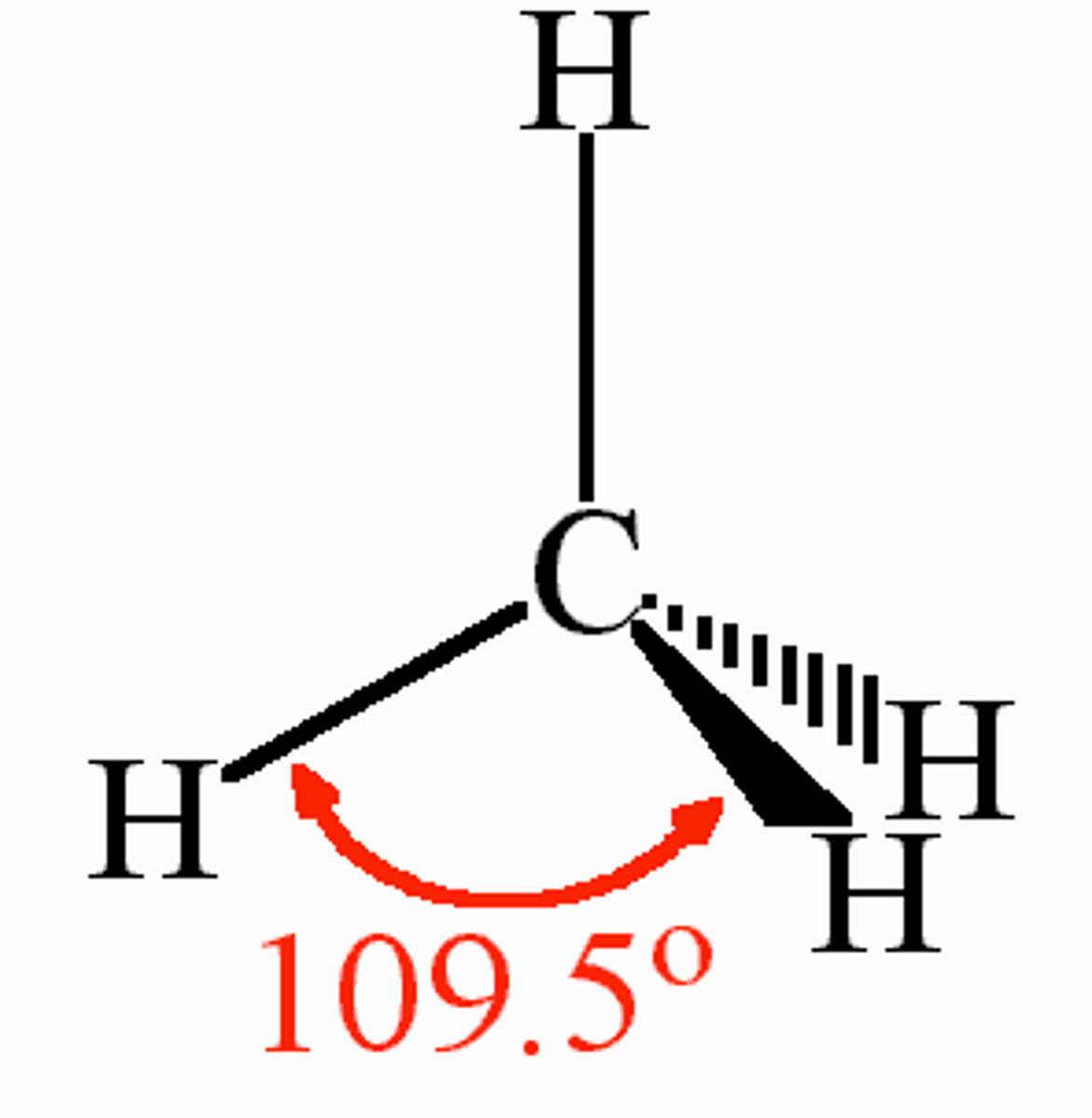
CRB What will the orbital geometry be for a carbon that is sp3 hybridized?
(A) Octahedral
(B) Trigonal bipyrmaidal
(C) Bent
(D) Tetrahedral
(D) Tetrahedral
A carbon that is sp3-hybridized will have tetrahedral orbital geometry.
True or False? σ Bonds can freely rotate while π Bonds cannot.
True. σ Bonds can freely rotate while π Bonds cannot.
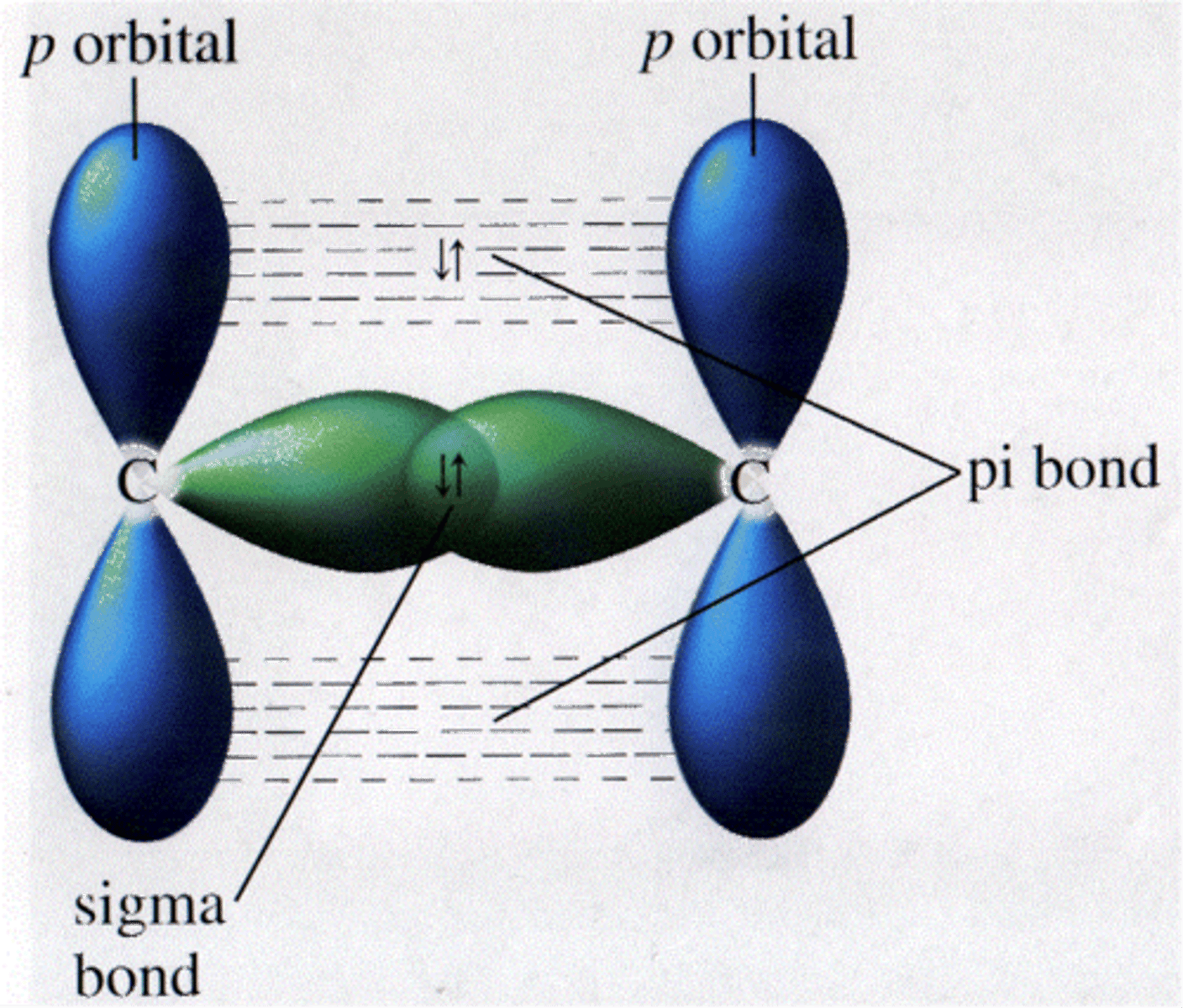
What is the purpose of the Steric Number?
It tells you how many hybridized orbitals you have in the central atom.
What equation is used to determine the steric number in terms of the number of σ Bonds in a compound?
SN = σ + LP
SN = Steric Number
σ = # of Sigma Bonds
LP = # of Lone Pairs
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
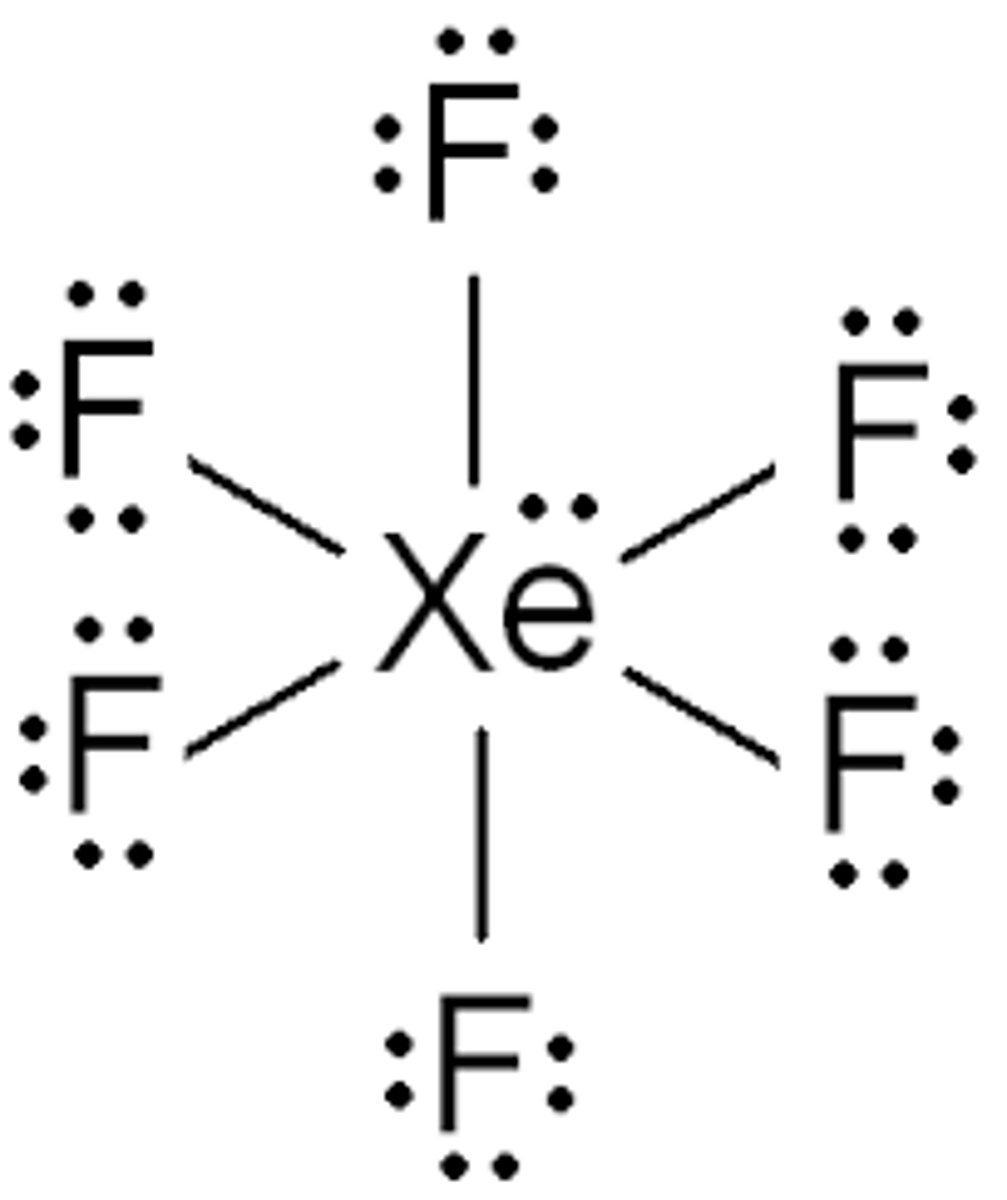
What is the steric number for XeF6 (periodic table: http://www.sbcs.qmul.ac.uk/iupac/AtWt/table.gif)?
(A) 5
(B) 6
(C) 7
(D) 8
(C) 7
SN = σ + LP
SN = 6 + 1
SN = 7
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is the equation for dipole moment (μ)?
μ = q d
μ = Dipole Moment
q = Charge
d = Distance
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
Cl- and H+ have partial charges of -4.76⋅10^-34 C and 4.76⋅10^-34 C, respectively. they are separated by a distance of 2.21⋅10^28 m. What is the dipole moment for HCl based on these numbers?
(A) 1.05⋅10^-5
(B) 4.62⋅10^-6
(C) 3.98⋅10^-7
(D) 9.45⋅10^-8
(A) 1.05⋅10^-5
μ = q d
μ = (4.76⋅10^-34 C)(2.21⋅10^28 m)
μ = approx. 10⋅10^-6 (actual: 10.52⋅10^-6)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
Compare the polarity of CCl4 and CHCl3. Why is the polarity the same/different?
CCl4 is non-polar due to its symetrical structure.
CHCl3 is polar due to its non-symetrical structure.
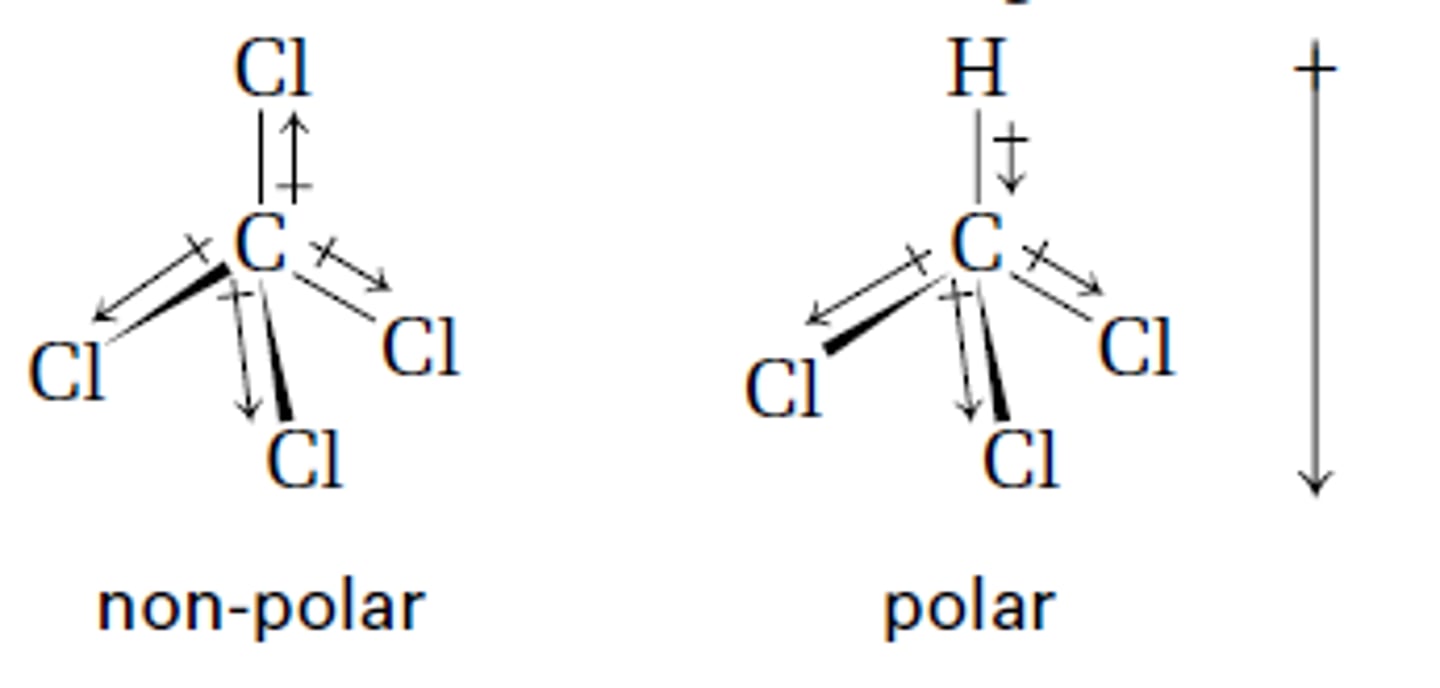
CRB Polar bonds can be used to stabilize Carbocations or Carbanions by donating or withdrawing electron density, respectively. Which of the following will be the most stable Carbanion?
(A) CH3
(B) CH2N
(C) CH2Br
(D) CCl3
(D) CCl3
The most stable Carbanion will have electron-withdrawing groups to stabilize the negative charge.
CRB Hey, CCl3 is a non-polar molecule! I thought you said that polarity is what will stabilize the carbanion? Please explain how this works.
For this stabilizing inductive effect to occur, there must be Polar Bonds, not necessarily a polar molecule! All that matters is that the carbanion has less electron density, not where the electron density moves to or if it is evenly distributed amongst the other atoms in the molecule.
CRB Polarity can be used to stabilize Carbocations or Carbanions by donating or withdrawing electron density, respectively. Which of the following will be the most stable Carbocation?
(A) CH3
(B) CH2N
(C) CH2Br
(D) CCl3
(A) CH3
To stabilize this carbocation, electron donating groups (read as Less Electronegative than Carbon) should be bound to Carbon. Nitrogen, Bromine and Chlorine are all more electronegative, so CH3 is the best answer.
CRB Another way to stabilize a carbocation or carbanion is by delocalizing the charge by having multiple resonance structures with the charge on different Carbons by moving double bonds. Which term properly describes this stabilizing property of multiple resonance structures?
(A) Inducibility
(B) Connectivity
(C) Allylic
(D) Conjugation
(D) Conjugation
Conjugation allows for delocalized charges by allowing a pi bond to exist between different (more than 2) adjacent carbons.
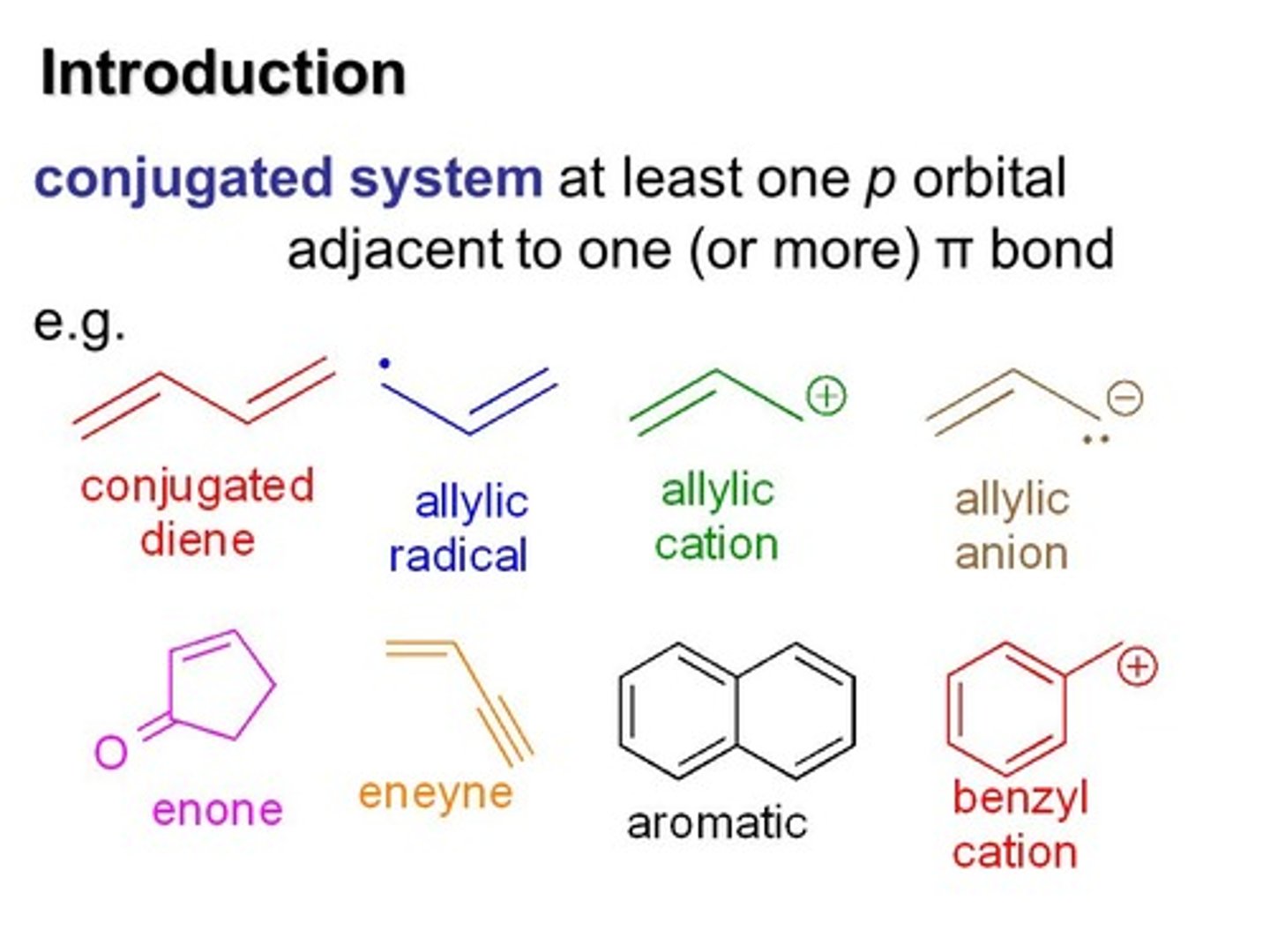
The Carbons of C2H4 need 3 hybrid orbitals each, yet they have only 2 orbitals with a single electron (and two orbitals with a lone pair of electrons). What will they do?
One of the 2s orbital electrons jumps up to fill the empty 2p orbital. Two of the three 2p orbitals then hybridize with the single 2s orbital, forming three sp2 orbitals and leaving one remaining p orbital. There is a single electron in each of these 4 described orbitals.
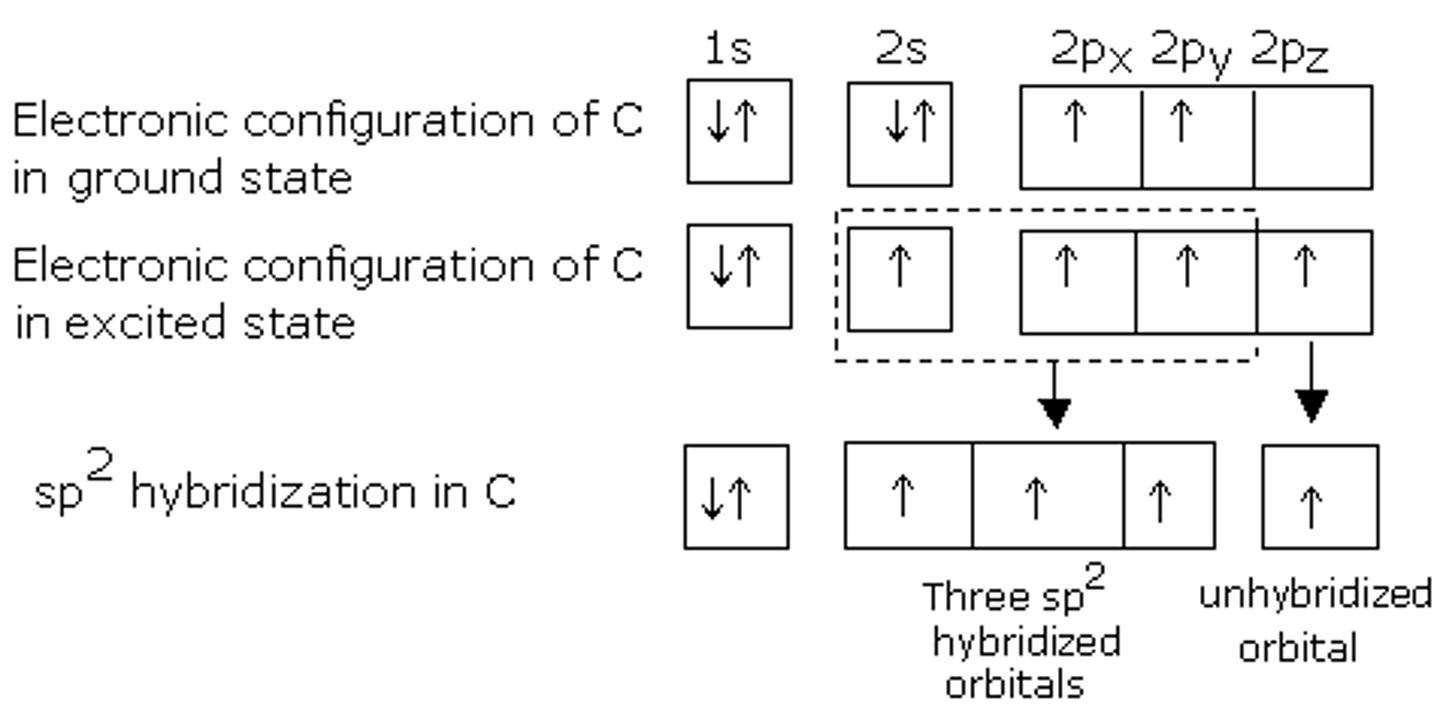
sp2 orbitals have what percent s character and what percent p character?
sp2 orbitals have 33 percent s character and 67 percent p character.
The sp2 orbitals will form _______ bonds while the p orbitals will form _______ bonds in making C2H4?
(A) σ,σ
(B) σ,π
(C) π,σ
(D) π,π
(B) σ,π
The sp2 orbitals will form σ Bonds with Hydrogens' s orbitals while the p orbitals will form π Bonds with each other in making C2H4.
CRB What will be the orbital geometry of a Carbon that is sp2-hybridized?
(A) Octahedral
(B) Bent
(C) Linear
(D) Trigonal Planar
(D) Trigonal Planar
A carbon that is sp2-hybridized will have a trigonal planar orbital geometry.
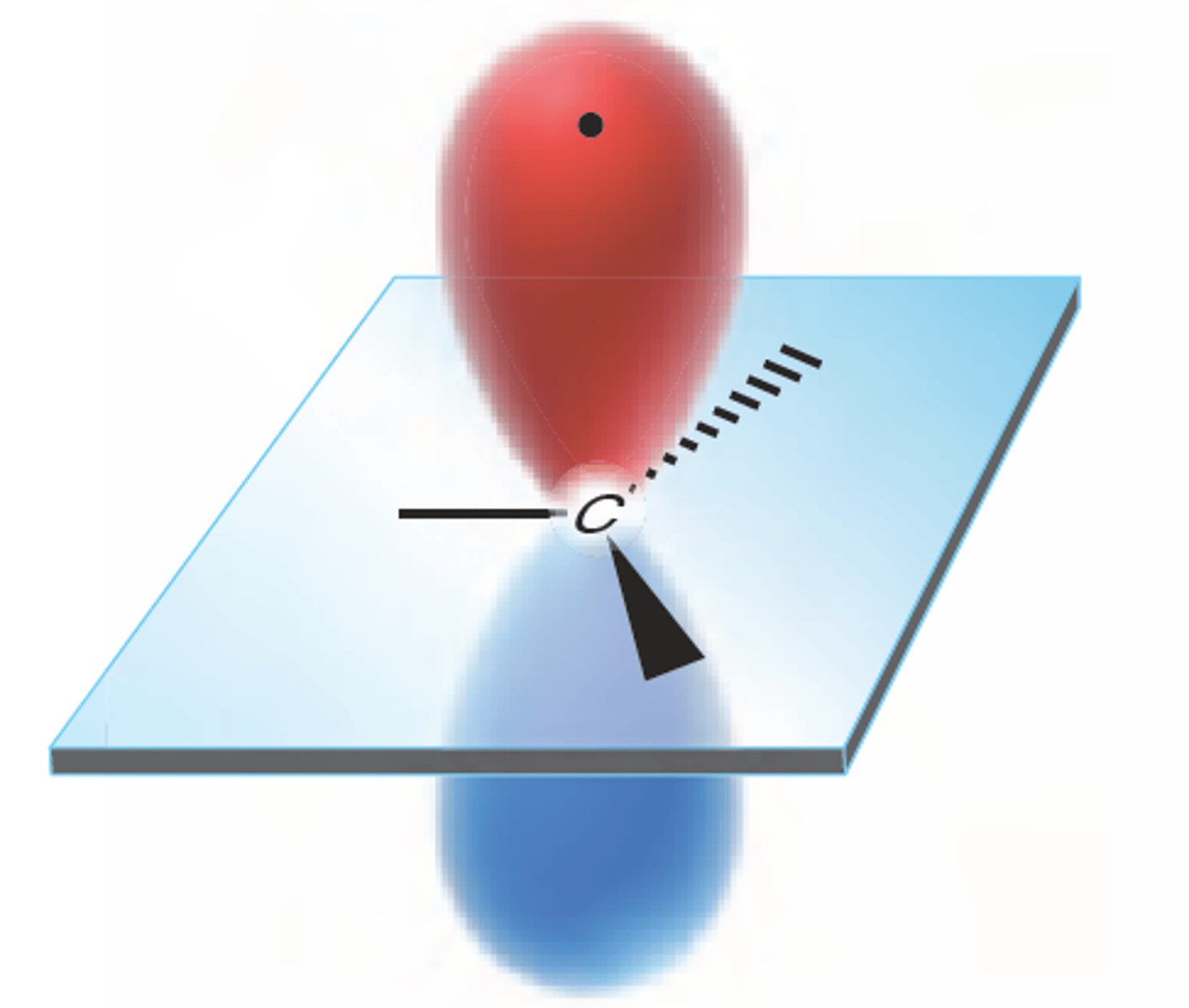
The Carbons of C2H2 only need 2 hybrid orbitals each, and they have 2 orbitals with lone pairs of electrons. What will they do?
One of the 2s orbital electrons jumps up to one of the 2p orbitals. One of the three 2p orbitals then hybridize with the single 2s orbital, forming two sp orbitals and two p orbitals with a lone electron in each.
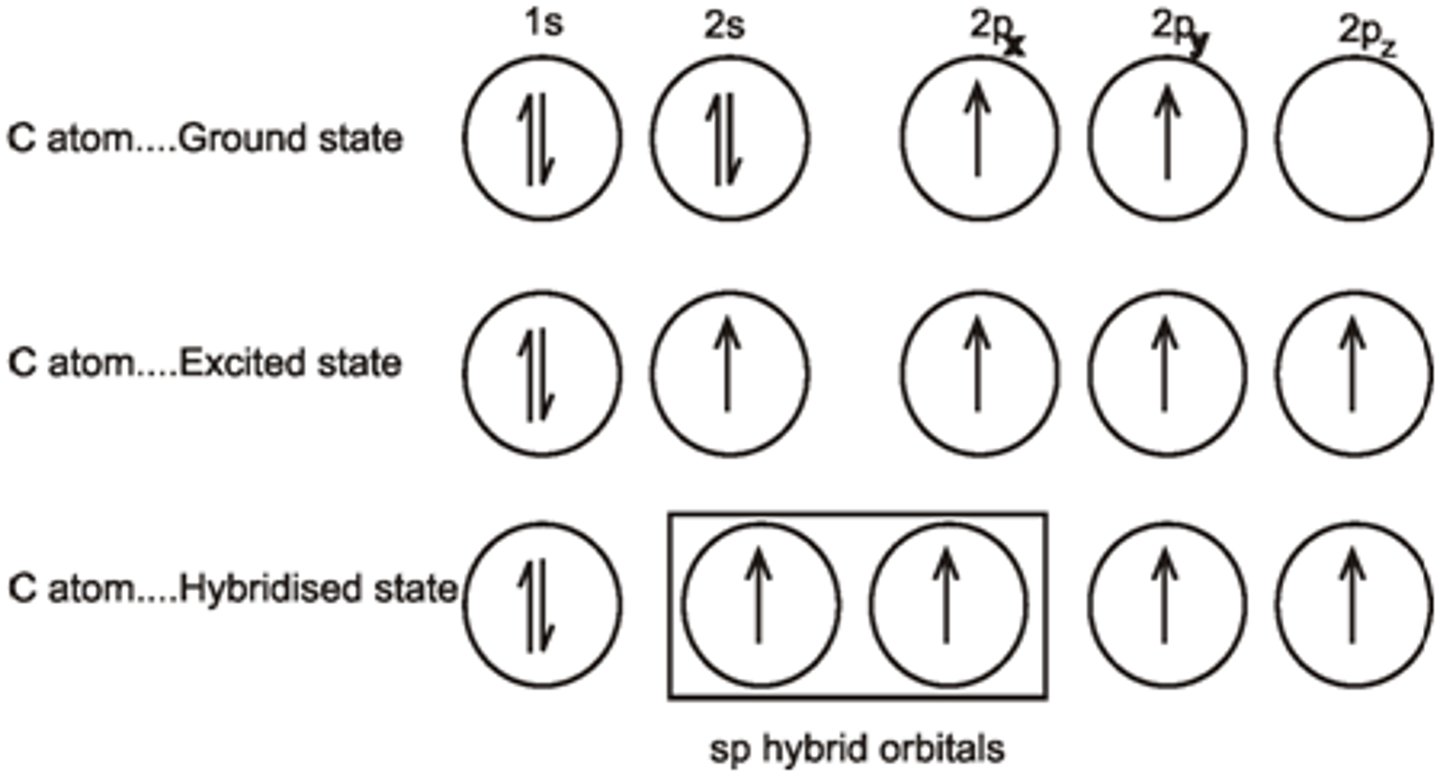
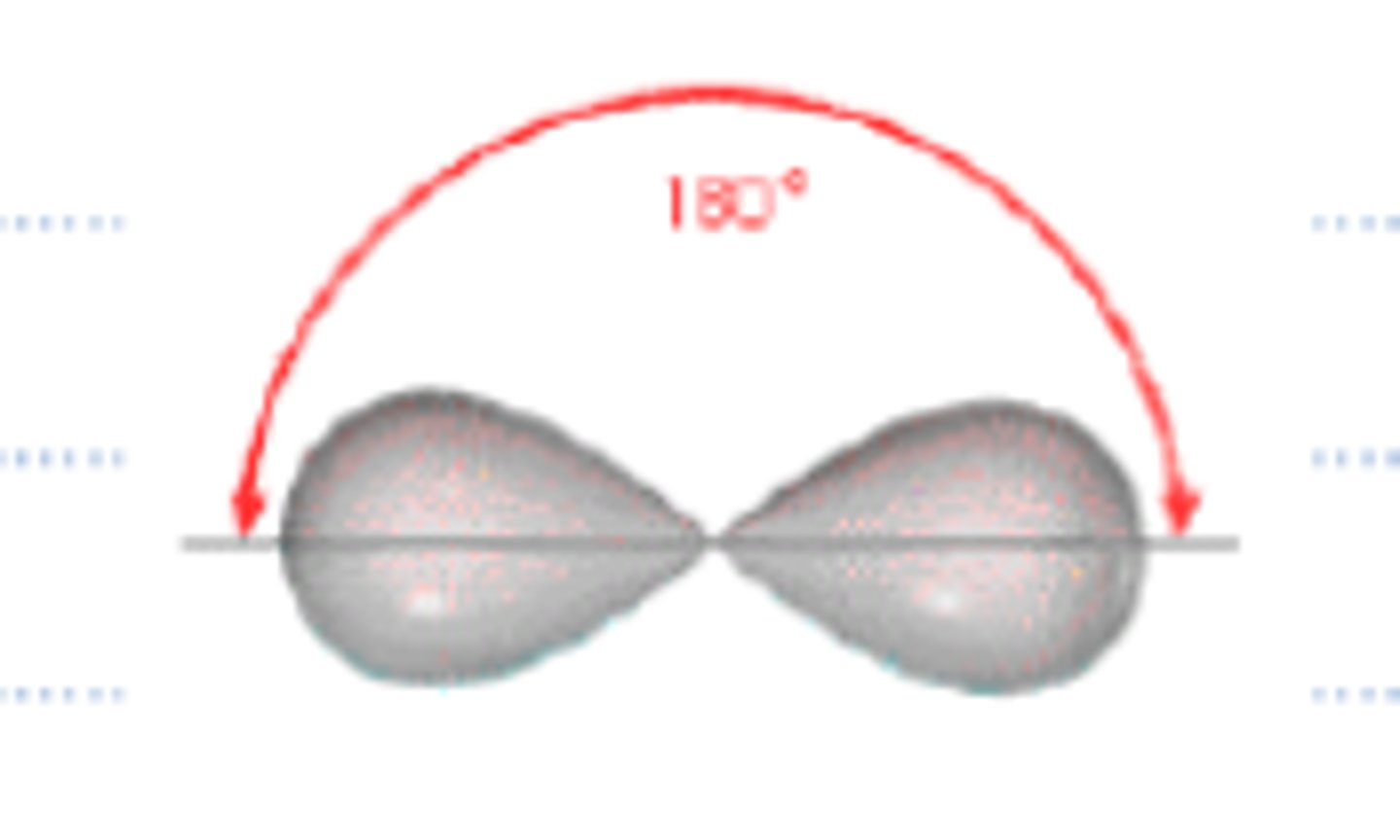
sp orbitals have what percent s character and what percent p character?
sp orbitals have 50 percent s character and 50 percent p character.
In C2H2, are the following bonds made of sp orbitals or p orbitals?
1. σ bonds between Carbon and Hydrogen
2. σ bond between Carbon and Carbon
3. π bonds between Carbon and Carbon
1. σ bonds between Carbon and Hydrogen - sp orbitals
2. σ bond between Carbon and Carbon - sp orbitals
3. π bonds between Carbon and Carbon - p orbitals
What will be the molecular geometry of a Carbon that is sp-hybridized?
(A) Octahedral
(B) Bent
(C) Linear
(D) Trigonal Planar
(C) Linear
An sp-hybridized Carbon will have a linear molecular geometry.
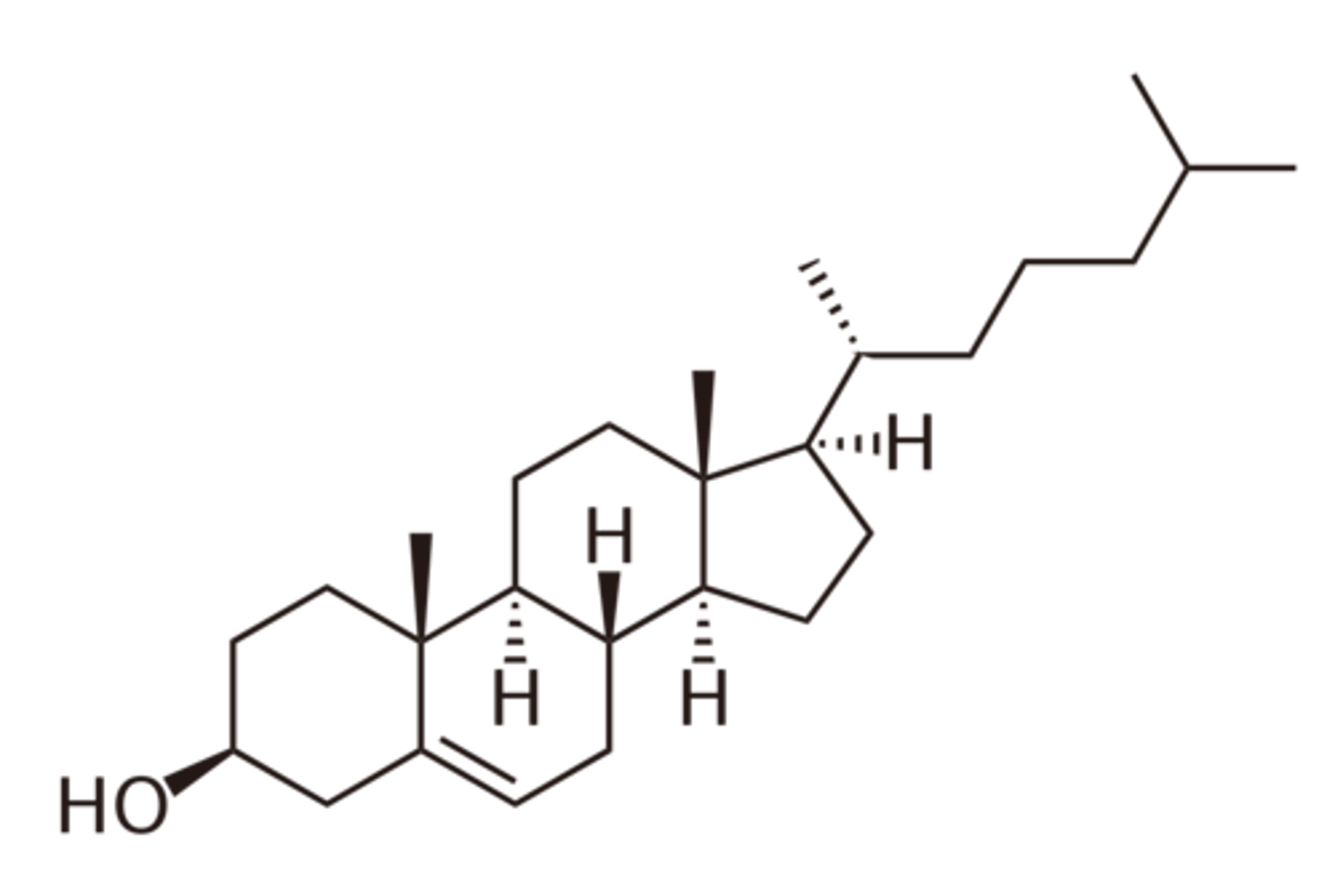
Cholesterol (https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=5997&t=l) has an important biochemical role in our bodies. How many sp3 carbons does it contain?
(A) 15
(B) 23
(C) 25
(D) 48
(C) 25
Cholesterol contains 25 sp3 hybridized carbons.
Do not forget the two carbons from wedges and one from dashes! Only the two carbons in the double bond are not sp3 hybridized here
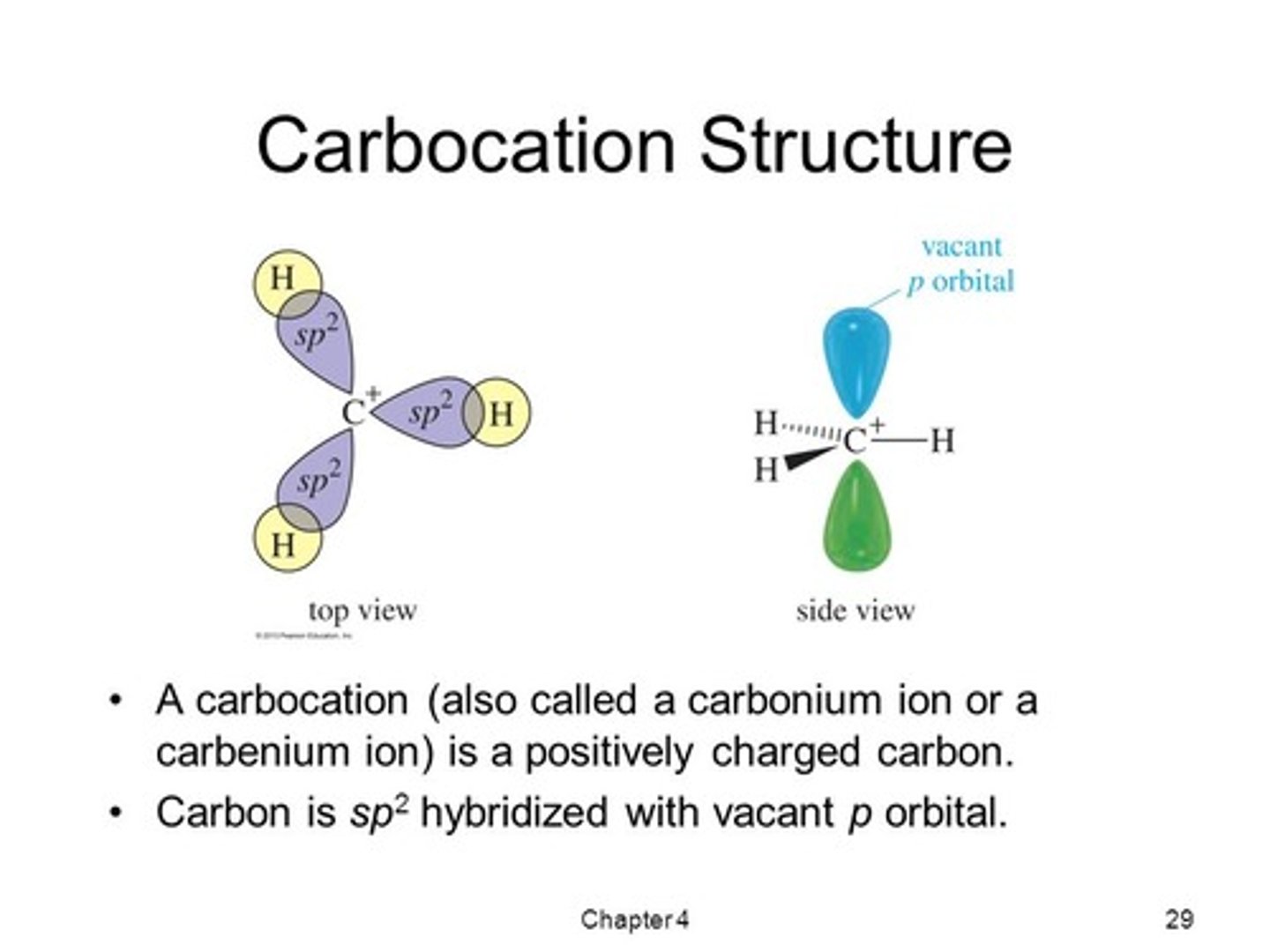
CRB A carbocation would take on the same orbital geometry of which of the following hybridizations?
(A) sp3
(B) sp2
(C) sp
(D) None of the above
(B) sp2
A carbocation has an empty p-orbital, and the other three orbitals will adopt the trigonal planar form that sp2-carbons use to maximize the space between each orbital.
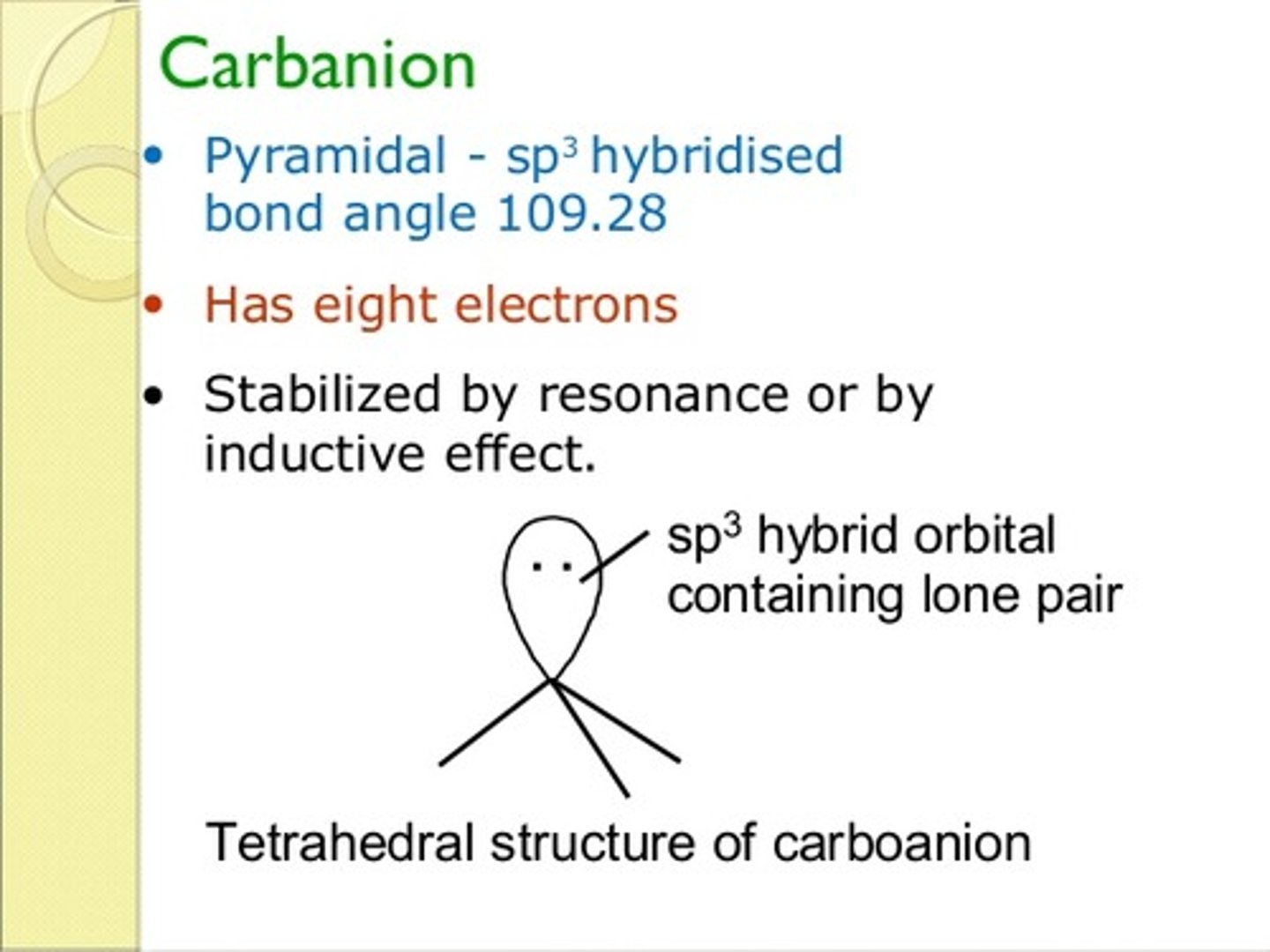
CRB True or false? Because both carbocations and carbanions have an unhybridized p-orbital, they will have the same orbital geometry.
False. Carbocations have a vacant p-orbital, so they can adopt a trigonal planar orbital geometry. Carbanions have a lone pair of electrons there that will repel the other three orbitals, preventing a trigonal planar orbital geometry.
CRB Based on the previous card, which of the following orbital geometries is most likely for a carbanion?
(A) Bent
(B) Tetrahedral
(C) Linear
(D) Trigonal Bipyramidal
(B) Tetrahedral
A carbanion takes on a slightly-distorted tetrahedral orbital geometry. That slight distortion is due to increased repelling from a lone pair than from two electrons in a covalent bond.
Note that the molecular geometry and orbital geometry will be different in this case.