2. Nucleotides and Nucleic Acids
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Pyrimidine base
6 membered heterocyclic ring with 2 N
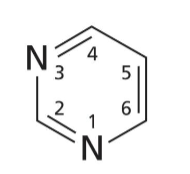
Common pyrimidines
Uracil, thymine, and cytosine
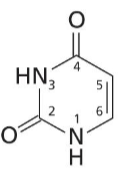
Uracil
Pyrimidine base with two O and two NH. Found in RNA
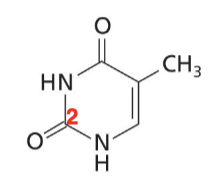
Thymine
Pyrimidine base with two O, two NH, and a CH3. Found in DNA
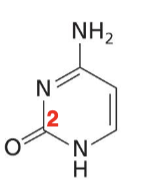
Cytosine
Pyrimidine base with one O, one N, one NH, one NH2
Purine base
6 membered and 5 membered heterocyclic rings with N on 1, 3, 7, and NH on 9
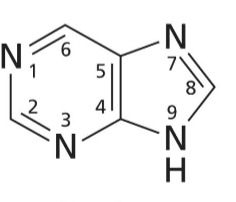
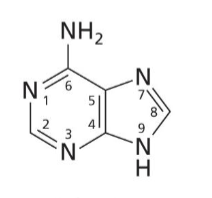
Adenine
Purine base with no O
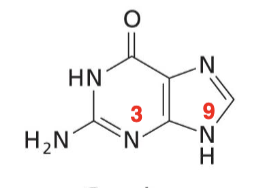
Guanine
Purine base with an O
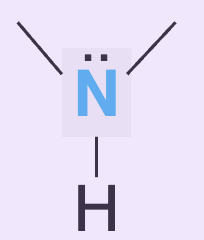
Lone pairs in resonance can accept hydrogen bonds. True or false?
False, the electrons are delocalized
Is the following lone pair involved in resonance? Can it accept hydrogen bonds?
Yes, no it can only donate.
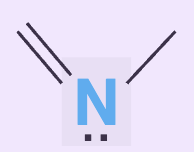
Is the following lone pair involved in resonance? Can it accept hydrogen bonds?
No, yes it can accept.
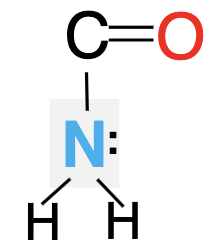
Is the following lone pair involved in resonance? Can it accept hydrogen bonds?
Yes, no it can only donate two.
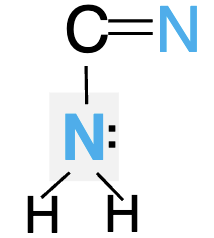
Is the following lone pair involved in resonance? Can it accept hydrogen bonds?
Yes, no it can only donate two.
Compare RNA vs DNA
RNA: polymer of G, A, C, U, ribose sugar, single stranded
DNA: polymer of G, A, C, T, deoxyribose sugar, double stranded
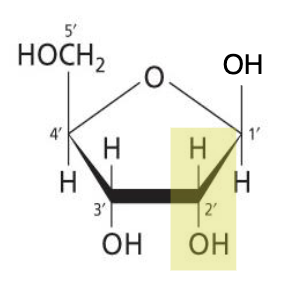
Compare structure of deoxyribose compared to ribose.
Deoxyribose is missing an O at C2’
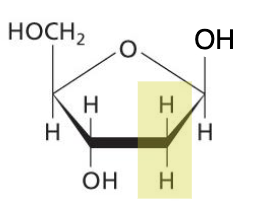
Nucleoside
Nitrogenous base attached to a pentose, 5C sugar.
How does the numbering of bases differ from sugars.
Sugars are numbered with primes, bases only use numbers
When a nucleoside is formed what parts of the sugar and nitrogen base are lost?
-OH lost from C1’ of sugar and H lost from N1 of base
In nucleoside formation, where is the sugar attached for pyrimidines vs purines.
N1 for pyrimidines N9 in purines
When naming nucleosides, how are purines named?
-ine becomes -osine (adenosine, guanosine)
When naming nucleosides, how are pyrimidines named?
ending is changed to -idine (cytidine, thymidine, uridine)
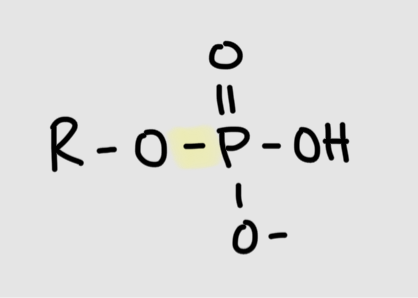
Formation of phosphoester bond
phosphate + hydroxyl group
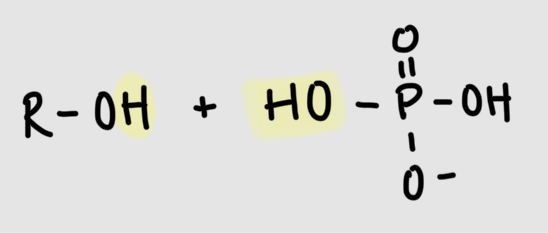
Nucleotide
Nucleoside + phosphate
How are nucleotides named
Nucleoside name + # of phosphates
Where doe the phosphates typically attach to nucleosides? Where else can they attach.
C5’. Can also attach to C2’ or C3’ (would need to be specified in the naming)
CONTINUE nucleic acids