unit 5 - electrochemistry
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
74 Terms
What is electrochemistry?
electrochemistry is the study of chemical processes that cause electrons to move
it involves chemical reactions that either absorb or release energy in the form of electricity
What is oxidation reaction vs reduction reaction? What is a redox reaction?
oxidation reaction
results in the loss of electrons and increase in oxidation number
reduction reaction
results in the gain of electrons and a decrease in oxidation number
Redox reaction
the net reaction involving both the reduction and oxidation reaction
What is an electrochemical cell?
any device that converts chemical energy to electrical energy or electrical energy to chemical energy
What are the three components needed to make up an electrochemical reaction?
There must be a solution where redox reactions can occur
A conductor must exist for electrons to be transferred
Ions must also be able to move through some form of salt bridge that helps with ion migration
What is the oxidation number of an atom? What do we assume the more electronegative atom bond does?
it is the charge the atom appears to have in a compound
we assume the more electronegative atom in a bond always gains electrons entirely, even if the bond is covalent
oxidation number:
C
0
oxidation number:
CO
C = +2
O = -2
oxidation number:
CO2
C = +4
O = -2
oxidation number:
CO32-
O = -2
C = +4
oxidation number:
ClO3-
O = -2
Cl = +5
oxidation number:
N2O
O = -2
N = +1
oxidation number:
NO
O = -2
N = +2
oxidation number:
N2O3
O = -2
N = +3
oxidation number:
NO2
O = -2
N = +4
oxidation number:
CuNO3

oxidation number:
CrO42-
O = -2
Cr = +6
oxidation number:
OF2
F = -1
O = +2
oxidation number:
Na2O2
Na = +1 (group 1 = +1)
O = -1 (peroxide)
oxidation number:
Fe(CN)2

oxidation number:
H2S
H = +1
S = -2
oxidation number:
Na2SO4
O = -2
Na = +1
S = +6
oxidation number:
SO2
O = -2
S = +4
oxidation number:
Na2S4O6
O = -2
Na = +1
S = -2.5
oxidation number:
SO
S = +2
O = -2
oxidation number:
SO2

oxidation number:
SO3
oxidation number:
H3PO2
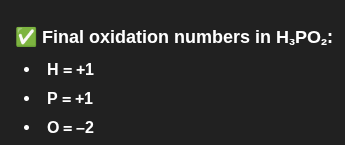
oxidation number:
H3PO3

oxidation number:
H3PO4

oxidation number:
H4P2O7

oxidation number:
H5P3O10

oxidation number:
Cs2O

oxidation number:
ClF3

oxidation number:
MoO42-

oxidation number:
PtCl62-

oxidation number:
H3AsO3

oxidation number:
MnO4-

oxidation number:
CaI2

oxidation number:
PtCl42-

oxidation number:
SnF2

oxidation number:
TiO2

oxidation number:
Al2O3

What is oxidizing agent vs reducing agent
Oxidizing agent: Substance that oxidizes another substance (opposite of reduced)
Reducing agent: Substance that reduces another substance (opposite of oxidized)
Write the:
substance oxidized
substance reduced
oxidizing agent
reducing agent
oxidation half reaction
reduction half reaction
Na(s) + H2O(l) →NaOH(aq) + H2(g)
Write the:
substance oxidized
substance reduced
oxidizing agent
reducing agent
oxidation reaction
reduction reaction
Cl2(g) + NaBr(aq) → Br2(g) + NaCl(aq)
Write the:
substance oxidized
substance reduced
oxidizing agent
reducing agent
oxidation reaction
reduction reaction
Fe2O3 + H2 → H2O + Fe

Write the:
substance oxidized
substance reduced
oxidizing agent
reducing agent
oxidation reaction
reduction reaction
H2 + O2 → H2O

Write the:
substance oxidized
substance reduced
oxidizing agent
reducing agent
oxidation reaction
reduction reaction
FeCl2 + Cl2 →FeCl3

Balance the redox reaction:
CuO + NH3 →Cu + N2
search in google
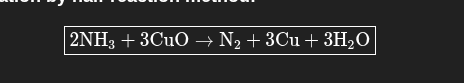
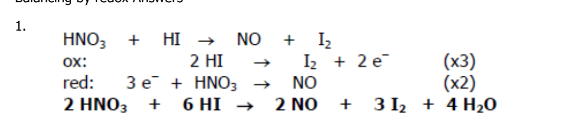
Balance the redox reaction:
HNO3 + HI →NO + I2
Balance the Redox reaction
Cu + HNO3 →Cu(NO3)2 + NO2
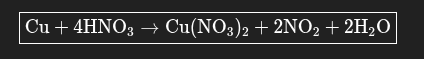
Balance the Redox reaction:
Cr2O72- + Cl- → Cr3+ + Cl2 (in basic solution)
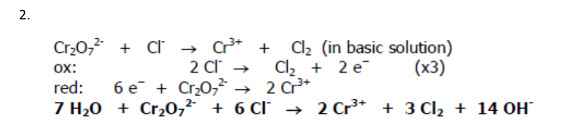
Balance the redox reaction:
CrO42- + SO32- → Cr3+ + SO42- (basic)
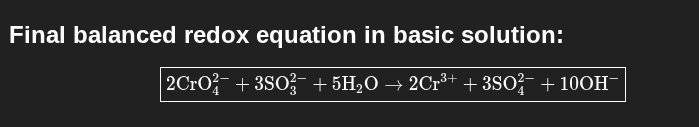
Balance the redox reaction:
MnO4- + C2O42- → Mn2+ + CO2 (acid)
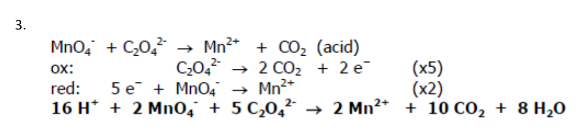
Balance the redox reaction:
Cl2 + IO3- → IO4- + Cl- (basic)
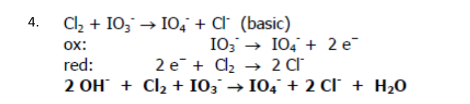
Balance the redox reaction:
NO3- + As2S5 → H3AsO4 + S + NO (acid)
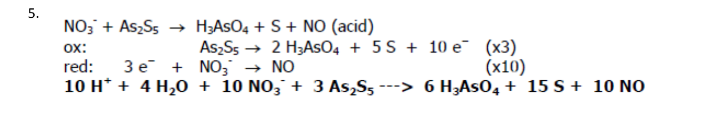
Balance the redox reaction:
Al + MnO4- → MnO2 + Al(OH)4- (basic MnO2)
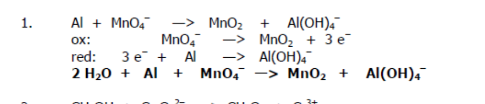
Balance the redox reaction:
CH3OH + Cr2O72- → CH2O + Cr3+ (acidic Cr2O72-)
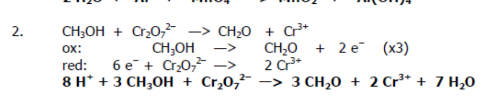
Balance the redox reaction:
NO2- + Al → NH3 + AlO2- (basic)
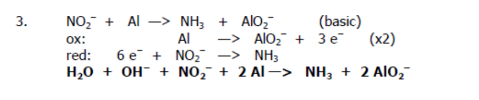
Balance the redox reaction:
CN- + MnO4- → CNO- + MnO2
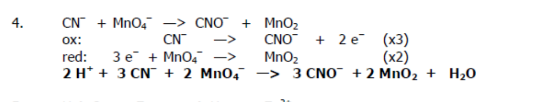

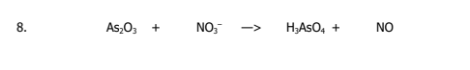
Balance
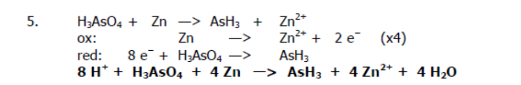

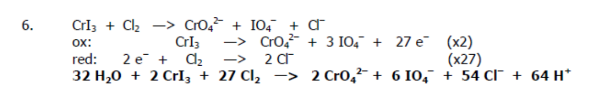

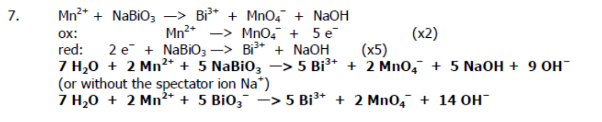
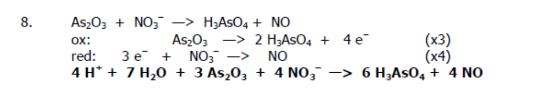
Recall the grade 11 activity series
17
Li
K
Ba
Ca
Na
Mg
Al
Zn
Fe
Ni
Sn
Pb
H
Cu
Ag
Hg
Au

Write the reaction, the oxidation half reaction, the reduction half reaction and the net ionic equation
Which is better at being reduced (which is the better oxidizing agent) Cu2+ or Fe2+?
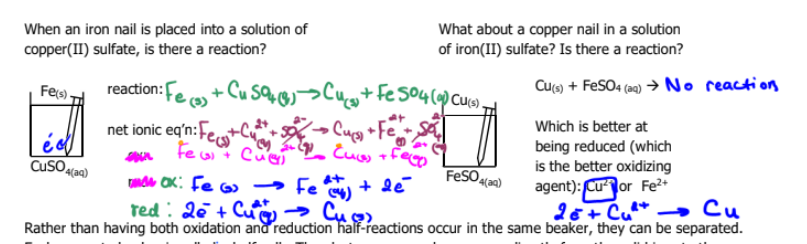
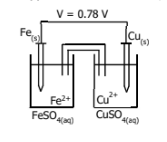
Which one is oxidation and which is reduction
and which one is anode and which one is cathode
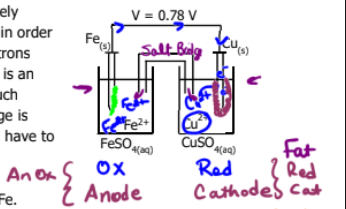

and do cell notation and label the diagram for red and ox and cathode and anode


and do cell notation and label the diagram for red and ox and cathode and anode

Draw an electrochemical cell for copper(II) nitrate and zinc nitrate
Label everything properly including reduction and oxidation and anode and cathode
Write down the half equations and write the net ionic equation
Do cell notation
Calculate the theoretical voltages


Draw an electrochemical cell for zinc nitrate and aluminum sulfate
Label everything properly including reduction and oxidation and anode and cathode
Write down the half equations and write the net ionic equation
Do cell notation
Calculate the theoretical voltages

