Transition Metal Info
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What is the definition of a transition element?
d-block element which forms at least one ion with a partially filled d orbital
What are the 3 properties of transition metals?
Variable oxidation states, form coloured compounds and can act as catalysts
What is a complex?
When one or more ligands form coordinate bonds to a central metal ion
What is a ligand?
A molecule or ion which donates a pair of electrons to a central metal ion forming a coordinate bond
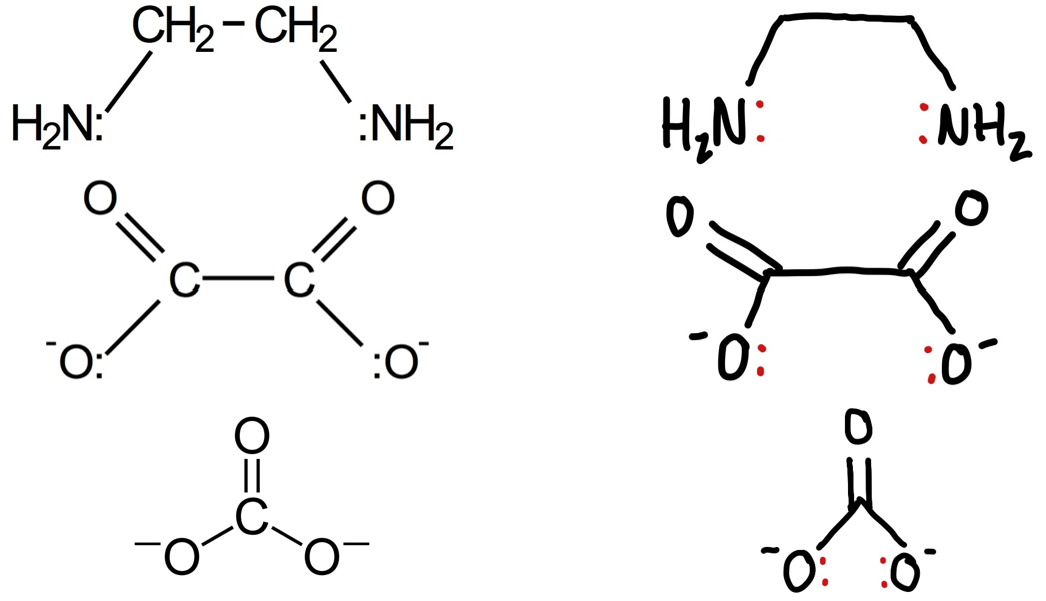
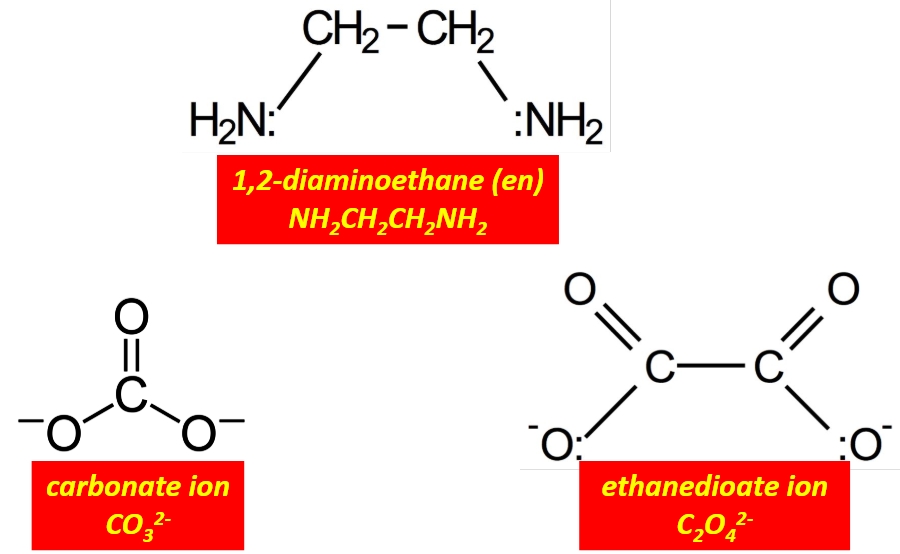
Give 3 examples of a bidentate ligand?
Ethandioate ion, carbonate ion or 1,2-diaminoethane
Which elements will form complex ions with a square planar shape?
Pt and Pd
In which kind of molecules does optical isomerism occur?
Octahedral complexes with 2 or more bidentate ligands
What colour is Cr(OH)3?
Grey-green