Part 5 - Intermolecular Forces of Attraction
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Between separate molecules, opposite attract. The more opposite, the more attraction.
a. Both true
b. Both false
c. Only 1st statement is true
d. Only 2nd statement is true
a. Both true
C is not significantly more electronegative than H.
a. True
b. False
a. True
Electrons are dispersing rapidly producing temporary partial positive and partial negative regions.
a. London dispersion forces
b. Dipole-Dipole
c. Ion-Dipole
d. Ion-Ion
a. London dispersion forces
Due to permanent dipoles between 2 molecules.
a. London dispersion forces
b. Dipole-Dipole
c. Ion-Dipole
d. Ion-Ion
b. Dipole-Dipole
Hydrogen bond is under which type of intermolecular forces?
a. London dispersion forces
b. Dipole-Dipole
c. Ion-Dipole
d. Ion-Ion
b. Dipole-Dipole
In hydrogen bond, partial negative charge comes from the H which is attached fo electronegative atoms such as O, N, and halogens.
a. True
b. False
b. False
H is the source of partial POSITIVE charge.
Hydrogen bond has higher positive charge with electronegative atoms such as
a. N
b. O
c. F
d. All
d. All
Hydrogen bond is stronger than usual type of dipole-dipole bond.
a. True
b. False
a. True
Contain strong hydrogen bond except:
a. N—H
b. O—H
c. C—H
d. F—H
e. None
c. C—H
C is not considered electronegative.
John Dalton (1803) proposed the:
a. Solid Sphere Model
b. Nuclear Model
c. Quantum Model
d. Planetary Model
e. Plum Pudding Model
a. Solid Sphere Model

J.J. Thomson (1904) proposed the:
a. Solid Sphere Model
b. Nuclear Model
c. Quantum Model
d. Planetary Model
e. Plum Pudding Model
e. Plum Pudding Model
Ernest Rutherford (1922) proposed the:
a. Solid Sphere Model
b. Nuclear Model
c. Quantum Model
d. Planetary Model
e. Plum Pudding Model
b. Nuclear Model
Niels Bohr(1913) proposed the:
a. Solid Sphere Model
b. Nuclear Model
c. Quantum Model
d. Planetary Model
e. Plum Pudding Model
d. Planetary Model
Erwin Schrodinger (1926) proposed the:
a. Solid Sphere Model
b. Nuclear Model
c. Quantum Model
d. Planetary Model
e. Plum Pudding Model
c. Quantum Model

Present in all molecules and atoms.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
a. Dispersion bonding
Present in polar molecules.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
b. Dipole-Dipole bonding
Present in electronegative atoms.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
b. Dipole-Dipole bonding
Present in molecules containing H bonded to F, O, or N.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
c. Hydrogen bonding
Present in mixtures of ionic and polar compounds.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
d. Ion-Dipole bonding
Strongest intermolecular force of attraction.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
d. Ion-Dipole bonding
Increasing strength
1) Dispersion bonding
2) Dipole-Dipole bonding
3) Hydrogen bonding
4) Ion-Dipole bonding
Weakest intermolecular force of attraction.
a. Dispersion bonding
b. Dipole-Dipole bonding
c. Hydrogen bonding
d. Ion-Dipole bonding
a. Dispersion bonding
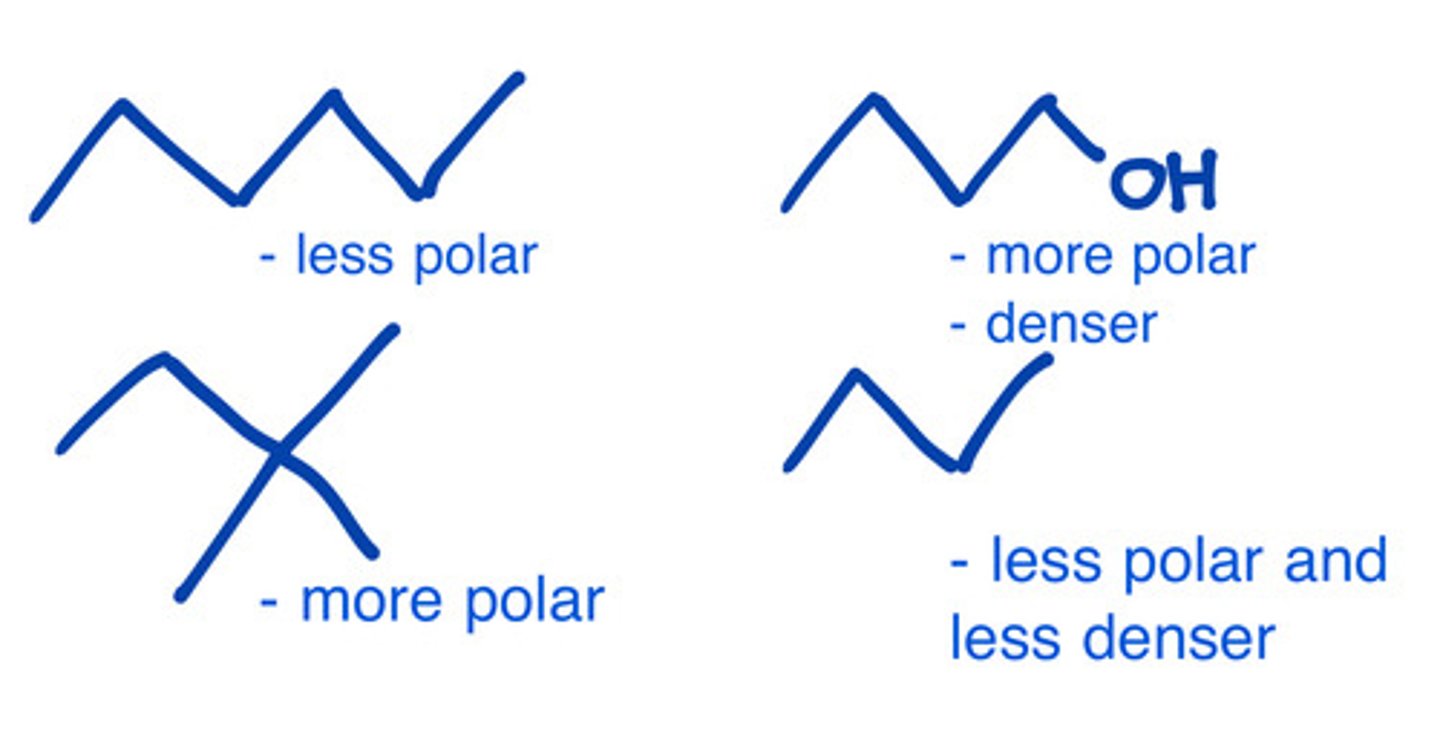
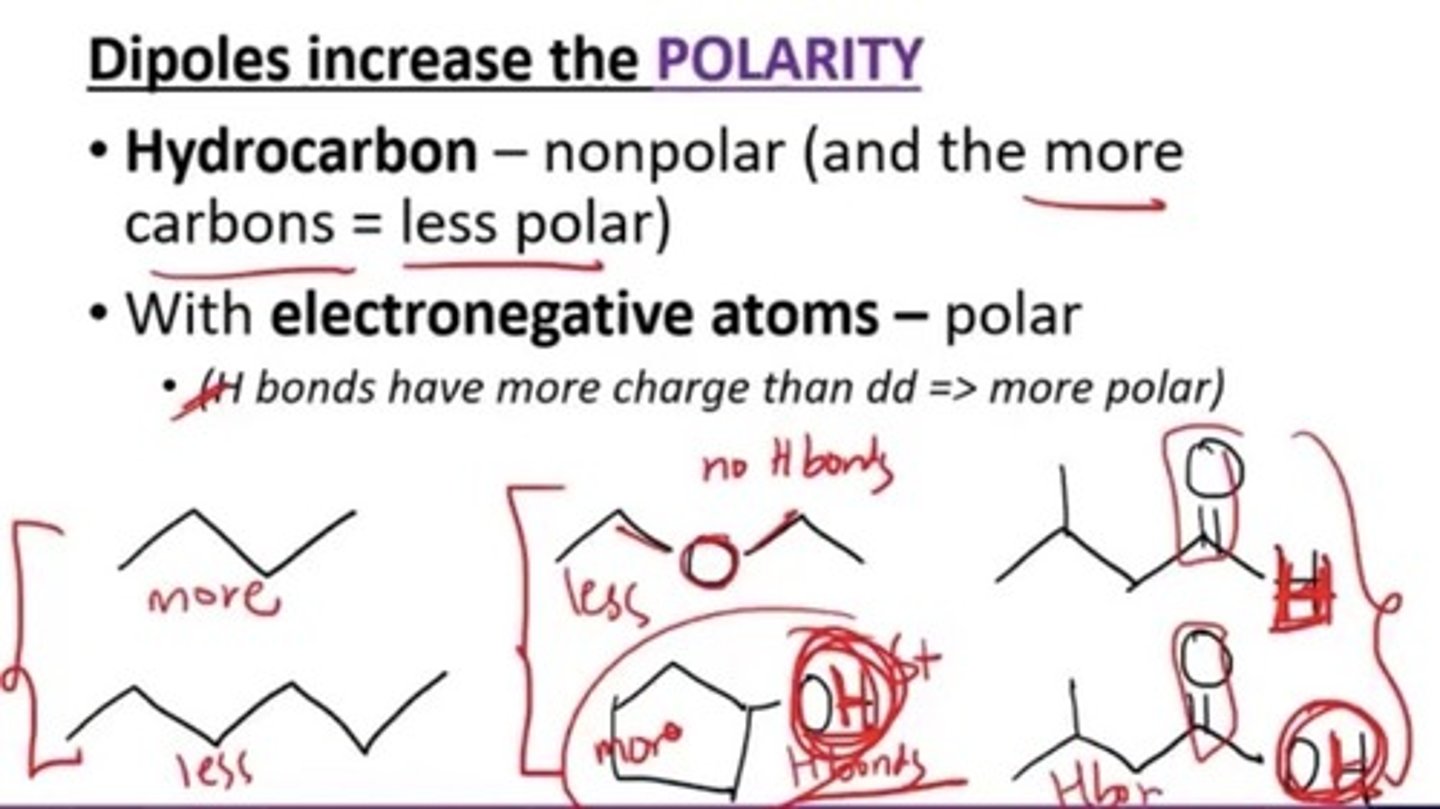
Which of the following increase the polarity of a compound?
a. More carbons
b. Presence of electronegative atoms
c. Presence of dipole or H bonding
d. a and b
e. b and c
f. All
e. b and c
*a) Hydrocarbons are non polar. More carbons make it more non polar.
b. Presence of electronegative atoms
c. Presence of dipole or H bonding
- H bonding will induce more polarity since its stronger
Solubility in water of CH3OCH3.
a. Soluble
b. Slightly soluble
c. Minimally soluble
d. Insoluble
a. Soluble - 2C ether is soluble to water.
Solubility in water of CH3OCH2CH3.
a. Soluble
b. Slightly soluble
c. Minimally soluble
d. Insoluble
a. Soluble - 3C ether is still soluble to water.
Solubility in water of CH3CH2OCH2CH3.
a. Soluble
b. Slightly soluble
c. Minimally soluble
d. Insoluble
b. Slightly soluble (10g/100g)
Solubility in water of CH3CH2OCH2CH2CH3.
a. Soluble
b. Slightly soluble
c. Minimally soluble
d. Insoluble
c. Minimally soluble (1.0g/100g)
Solubility in water of CH3CH2CH2OCH2CH2CH3.
a. Soluble
b. Slightly soluble
c. Minimally soluble
d. Insoluble
d. Insoluble (0.25g/100g)
Which of the following increases the water solubility?
a. Presence of dipoles
b. Presence of H bonds
c. Presence of branching
d. a and b
e. b and c
f. All
f. All
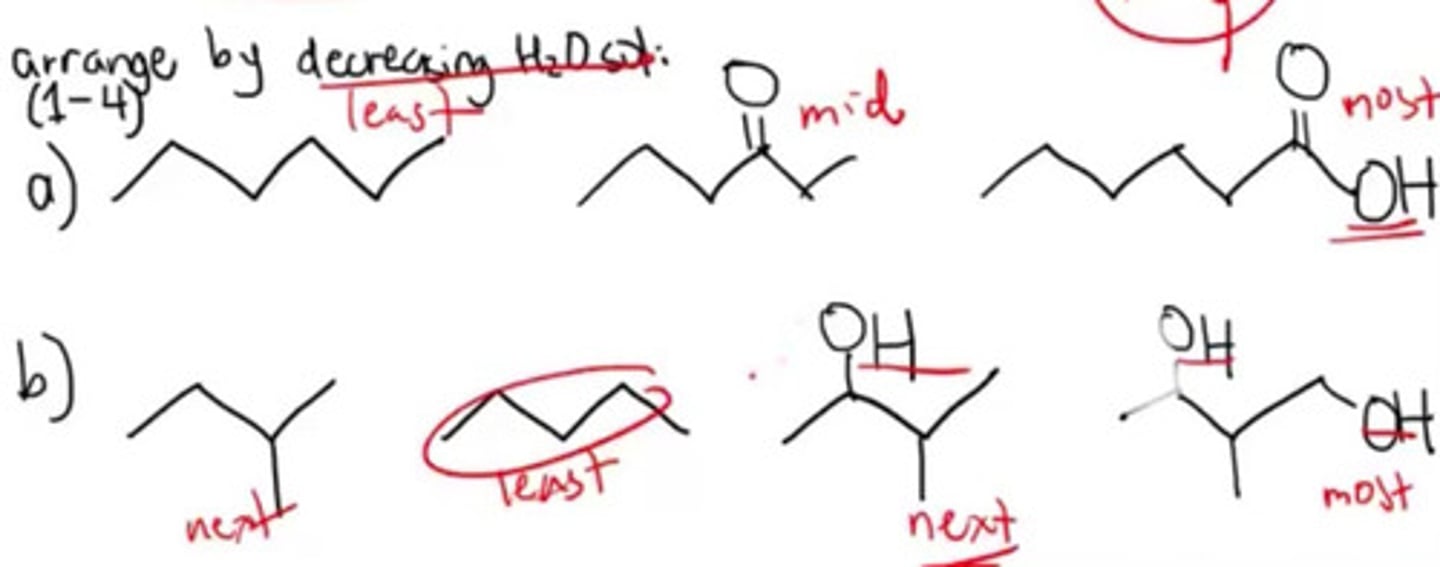
Increasing induction of water solubility of different bonds.
a. London bond —> Dipole-dipole bond —> Hydrogen bond
b. London bond —> Hydrogen bond —> Dipole-dipole bond
c. Hydrogen bond —> London bond —> Dipole-dipole bond
d. Hydrogen bond —> Dipole-dipole bond —> London bond
a. London bond —> Dipole-dipole bond —> Hydrogen bond
Presence of hydrogen bond will make the most water soluble.
Which of the following affects the intermolecular force attraction?
a. Contact area between molecules
b. Surface area of molecules
c. Quantity of molecule in contact
d. a and b
e. All
e. All
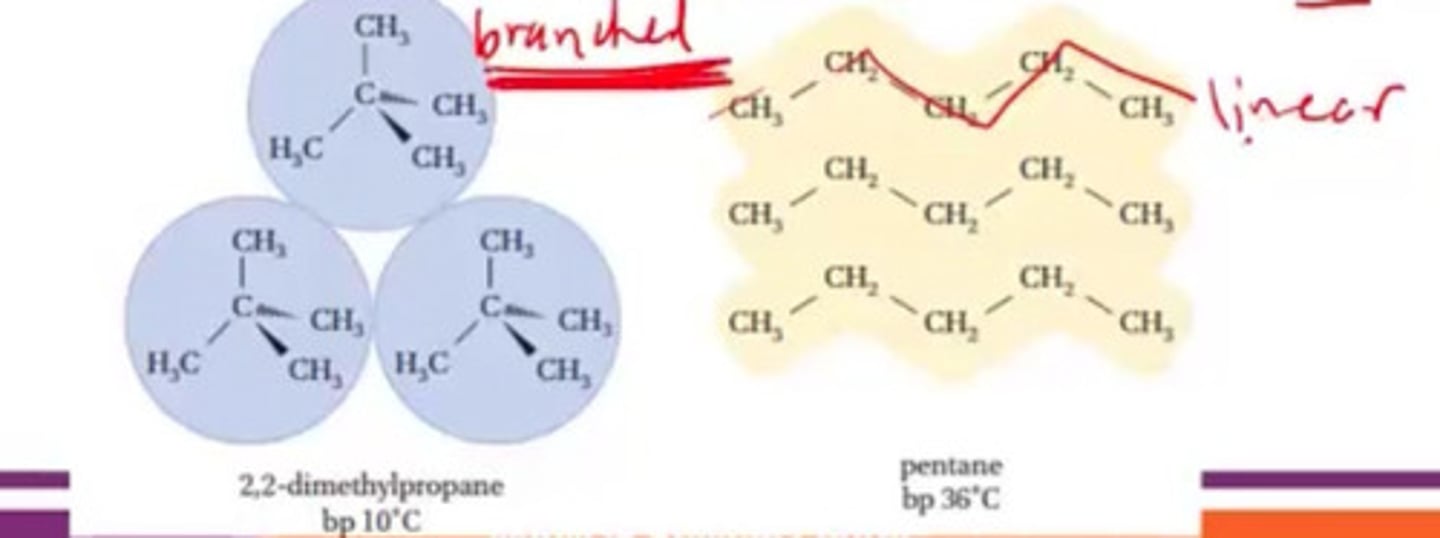
Which will decrease IMF?
a. Linear
b. Branched
c. Both
d. None
b. Branched - It will reduce stacking decreasing good contact.
Which will increase IMF?
a. Linear
b. Branched
c. Both
d. None
a. Linear - It will lead to good stacking which improve contact between molecules

Which of the following will be increased by stronger IMF?
a. Boiling point
b. Melting point
c. Density
d. a and b
e. b and c
f. All
f. All
Effects of presence of electronegative atoms to boiling point, melting point, and density.
a. Increase
b. Decrease
c. No change
a. Increase
Effects of more carbons to boiling point, melting point, and density.
a. Increase
b. Decrease
c. No change
a. Increase - More carbons means more bonding which will result to relatively stronger IMF.
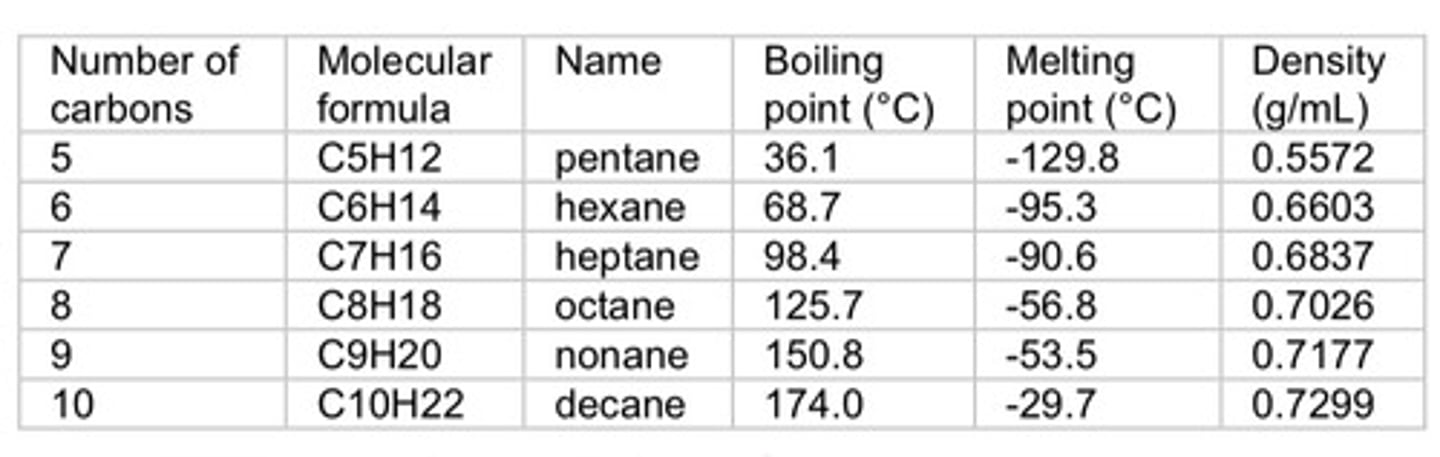
Effects of more branching to boiling point, melting point, and density.
a. Increase
b. Decrease
c. No change
b. Decrease - Branching decrease IMF thus lowers boiling point, melting point, and density.