Ch 13 Intro to Metabolism
1/123
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
124 Terms
Life needs what?
Energy
Living structures are built of what? (broad term)
Complex structures
How is building of complex structures that are low in entropy possible?
Energy is spent in the process
The ultimate source of energy that life on Earth uses is what?
Sunlight
In energy cycling what type of organisms directly use sunlight?
Photosynthetic autotrophs
What do photosynthetic autotrophs produce?
Oxygen (O2) and organic products such as glucose and other carbohydrates
What is metabolism?
The sum of all chemical reactions in a cell
A typical eukaryotic cell has the capacity to make approximately how many different proteins?
~30,000 different proteins
What two functions do proteins primarily have in a cell?
Structural and enzymes
Can metabolites be shared by more than one “pathway”?
Yes, most are shared
What do heterotrophs produce?
CO2, H2O, and ATP (energy)
There are thousands of different reactions that can occur in a cell how many metabolites can some of these “pathways” have?
Many hundreds
Catabolic pathways deliver what? In the form of what? How are these deliveries used?
Catabolic pathways deliver chemical energy in the form of ATP, NADH, NADPH, and FADH2. These energy carriers are used in anabolic pathways to convert small precursor molecules into cellular macromolecules.
Are anabolic pathways synthesizing or degrading?
Synthesizing
Are catabolic pathways synthesizing or degrading?
Degrading
Conversion from energy-containing nutrients such as carbohydrates, fats, and proteins, to energy-depleted products such is CO2, H2O, and NH3 coupled with the release of chemical energy is what type of process?
Catabolism
When electron carriers like NAD+, NADP+, and FAD go through a catabolic process are they oxidized or reduced?
Reduced to NADH, NADPH, and FADH2
The use of precursor molecules like amino acids, sugars, fatty acids, and nitrogenous bases to build cell macromolecules such as proteins, polysaccharides, lipids, and nucleic acids through the use of chemical energy is what type of process?
Anabolism
When electron carriers like NAD+, NADP+, and FAD go through a anabolic process are they oxidized or reduced?
Oxidized
A pathway that is dual-purpose that both generates energy (catabolic) and provides precursor molecules for biosynthesis (anabolic) is called what?
Amphibolic pathway (The citric acid cycle is an example of this type of pathway)
Enzymes are regulated by one mechanism or many?
Many
Can any gene directly make animo acids?
No, genes can make enzymes that produce amino acids which can be regulated up to down as needed.
What is the definition of of a metabolic pathway?
A series of consecutive enzymatic reactions that result in a product that is either used or excreted by the cell. MULTIPLE REACTIONS!
What are the 3 major forms in a metabolic pathway
Precursors → Intermediates (Metabolites) → Product
Do catabolic pathways converge or diverge?
Converge
Do anabolic pathways converge or diverge?
Diverge
What do catabolic pathways converge towards and anabolic pathways diverge from? What key central metabolite?
Acetate (acetyl-CoA)
Do chemical changes and energy transductions in living organisms follow the laws of thermodynamics?
Yes
Define the free-energy change in terms of ability to do work
When a chemical reaction occurs the free-energy change is the maximum energy made available to do work
When combining two reactions to yield a third reaction how is the overall free energy change calculated?
A sum of the two reactions free energy changes
Cells accomplish energy-requiring chemical work how?
By coupling an energy-releasing (exergonic) reaction, like cleavage of ATP, to an endergonic reaction (which requires energy input)
When describing the thousands of different chemical reactions that occur in the biosphere they can fall into a large or small set of reaction types?
Small
What is the universal energy currency in living organisms?
ATP
Why is ATP the universal energy currency?
Transfer of its phosphoryl group to a water molecule or metabolic intermediates provides the energetic push for muscle contraction, the pumping of solutes against concentration gradients, and the synthesis of complex molecules.
What type of reactions indirectly provide much of the energy needed to make ATP? And how?
Oxidation-reduction reactions. Reduced substrates like glucose are oxidized in several steps, with the energy of oxidation steps conserved in the form of a reduced cofactor, NADH. Energy stored in NADH is used to drive the synthesis of ATP.
What are two major mechanisms by which cells regulate enzymes?
Change the number of enzyme molecules
Change the catalytic activity of preexisting enzyme molecules
What 3 statements about living organisms use of energy is universally and thermodynamically always true?
Living organisms cannot create energy from nothing
Living organisms cannot destroy energy into nothing
Living organisms may transform energy from one form to another
In the process of transforming energy living organisms must increase or decrease the entropy of the universe
Increase
In order to maintain organization within themselves, living systems must be able to do what?
Extract usable energy from their surroundings and release useless energy (heat) back to their surroundings
Is delta G positive or negative if Keq is > 1.0. Is the reaction spontaneous?
Negative. Yes a spontaneous reaction.
Is delta G positive or negative if Keq is < 1.0. Is the reaction spontaneous?
Positive. Reaction is non-spontaneous.
What is the relationship between Keq and delta G?
Exponential
What is Keq?
[Products] / [Reactants]
Hydrolysis of one ATP can generate ~30kJ of energy which can increase product formation of a reaction by how much?
10^6 or an increase of 1,000,000x
When Keq is 1.0 what is delta G equal to? What does this mean about the reaction?
Zero. The reaction is at equilibrium.
What is the standard free energy change assumption regarding concentration of components?
All components start at 1M
The ACTUAL free-energy change of a reaction in the cell depends on what?
The standard free energy change
Actual concentrations of products and reactants
Standard free energy changes in a pathway way are totaled how?
Additive
How can thermodynamically unfavorable (endergonic) reactions be driven in the forward directions?
By coupling to a highly exergonic (energetically favorable) reaction. Often ATP hydrolysis.
Approximately how much free energy is released from hydrolysis of ATP under standard conditions?
-30.5 kJ/mol
How does ATP hydrolysis drive metabolism?
Shifts the equilibrium of coupled reactions
How are Keq (equilibrium constants) for coupled reactions associated together?
Multiplicative
How are delta G for coupled reactions associated together?
Additive
What does a positive Keq mean? More product or more reactant?
More product than reactant
What does a negative Keq mean? More product or more reactant?
More reactant than product
Why is hydrolysis of ATP highly favorable under standard conditions? (3 major reasons)
Electrostatic repulsion
Removal of phosphate group takes away a negative repulsive charge, more stable product
Resonance stabilization
ADP and Pi, have a greater resonance stabilization than ATP. Electrons are shared better.
Stabilization due to hydration (cells are aq. environments)
More water can bind more effectively to ADP or Pi, than ATP, thereby stabilizing ADP and Pi
At pH 7.0 what charge does ATP carry?
-4
The Delta G of ATP hydrolysis is dependent on what? How does it regulate ATP hydrolysis?
ATP hydrolysis is Mg++ dependent. ATP in a cell always has Mg++ bound which prevents spontaneous hydrolysis by partially shielding negative charges and influencing the conformation of phosphate groups.
Mg++ from where binds to ATP and ADP?
The cytosol
Enzymes which participate in phosphate transfers and have an affinity for Mg are known as what?
Kinases
What is the true substrate in most enzymatic reactions involving ATP?
MgATP
When ATP is involved in a reaction how does it affect the product?
It phosphorylates the product
What is phosphorylation potential (delta Gp)?
The actual free energy of hydrolysis of ATP under intracellular conditions which varies from cell to cell and over time based on available concentrations
In vivo (in the cell), the energy release by ATP is greater or less than the standard free-energy change?
Greater
Are there biosynthetic reactions that use NTPs other than ATP such as UTP, GTP, and CTP? How much energy does hydrolysis of these NTPs release under standard conditions?
Yes other NTPs are required. They release a very similar amount of energy as ATP, -30.5kJ/M
Are the concentrations of ATP, ADP, and Pi every actually all 1M?
No
Are the free concentrations of ATP the same as the actual concentrations of ATP?
No, ATP can be found bound to several proteins.
What is an essential form of cellular energy transformation?
Phosphoryl transfer potential
What 3 molecules have a higher phosphoryl transfer potential (free energy change due to hydrolysis) than ATP?
1,3-Bisphosphoglycerate
Phosphoenolpyruvate
Phosphocreatine
Acetyl-Coenzyme A has what type of high energy bond?
Thioesters
Are thioesters more or less stable than oxygen esters? Why?
Less. Oxygen is stabilized by resonance.
How does ATP provide energy?
Group transfers
What are the two major steps by which ATP provides energy?
Transfer part of ATP molecule to substrate or amino acid residue activating it
Displacement of the phosphate-containing moiety generating Pi, PPi, or AMP as the leaving group
Most reactions in biochemistry are what type of process? Heterolytic or homolytic?
Heterolytic
Nucleophiles react with what?
Electrophiles
Heterolytic bond breakage often gives rise to what?
Transferable groups, such as protons
Oxidation of reduced fuels often occurs via what type of process?
Transfer of electrons and protons to a dedicated redox cofactor
Are nucleophiles electron rich or poor? What type of charge do they react with?
Electron rich and react with positive charges
Are electrophiles electron rich or poor? What type of charge do they react with?
Electron poor (positively charged) and react with negative charges
What 6 reaction categories do most reactions fall into? If applicable what mechanisms do they use?
Cleavage and formation of C-C bonds
Homolytic cleavage (very rare)
Heterolytic cleavage (most common)
Cleavage and formation of polar bonds
Nucleophilic substitution mechanism
Addition-elimination mechanism
Hydrolysis
Condensation reactions
Internal rearrangements
Eliminations (without cleavage)
Group transfers (H+, CH3+, PO3²-)
Oxidation-reductions (e- transfers)
Define homolytic cleavage
Cleavage of C-C or C-H bond where each atom keeps one of the bonding electrons, resulting in the formation of carbon radicals or uncharged hydrogen atoms. Equal distribution of electrons.
Define heterolytic cleavage
Clevage of a C-C or C-H bond where one of the atoms retains both of the bonding electrons. Leads to formation of carbon ions which are highly unstable.
Carbon ions are so unstable that they are unable to be captured by what?
Enzymes
What are three functional groups that can help to stabilize carbon ions and facilitate their formation as intermediates?
Carbonyl group - electrophilic
Imine group - electrophilic
Carbonyl groups augmented by a metal ion or general acid (HA)
What are 3 reaction types that are an example of nucleophilic C-C bond formation reaction?
Aldol condensation - carbanion serves as nucleophile and carbon of carbonyl serves as electrophile
Claisen ester condensation - carbanion stabilized by carbonyl of an adjacent thioester
Decarboxylation of a Beta-keto acid - carbanion forms as the CO2 leaves
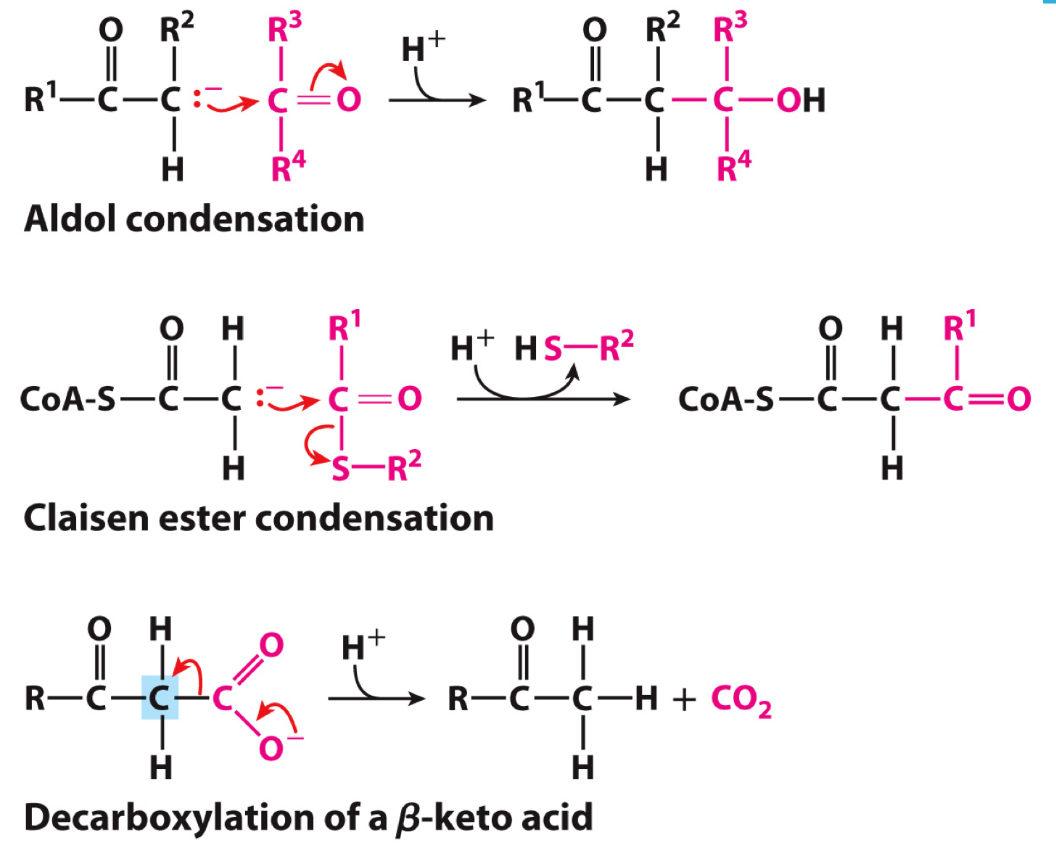
In each case of nucleophilic C-C bond formation the carbanion is stabilized by what?
Carbonyl at the adjoining carbon
In internal rearrangement, isomerization and elimination is there a change in the oxidation state? What are 3 other potential changes that can occur?
No change in oxidation state.
Different groups can undergo oxidation and reduction
Group at double-bond undergo cis → trans rearrangements
Position of double-bond is transposed
What enzyme is involved in changing Glucose 6-phosphate to Fructose 6-phosphate? What type of intermediate is involved in this process?
Phosphohexose isomerase and an enediol intermediate
(aldose → ketose)
What are the 5 steps in changing Glucose 6-phosphate to Fructose 6-phosphate (Aldose → Ketose)
B1 abstracts a proton (acts as a base)
Formation of C=C
e- from carbonyl form O-H w/ H donated by B2
B2 abstracts proton, allowing formation of C=O bond
e- pair is displaced from C=C bond to form C-H bond w/ proton donated by B1
(Step 4 & 5 occur in enediol intermediate)
(B1 and B2 are ionizable groups on the enzyme)
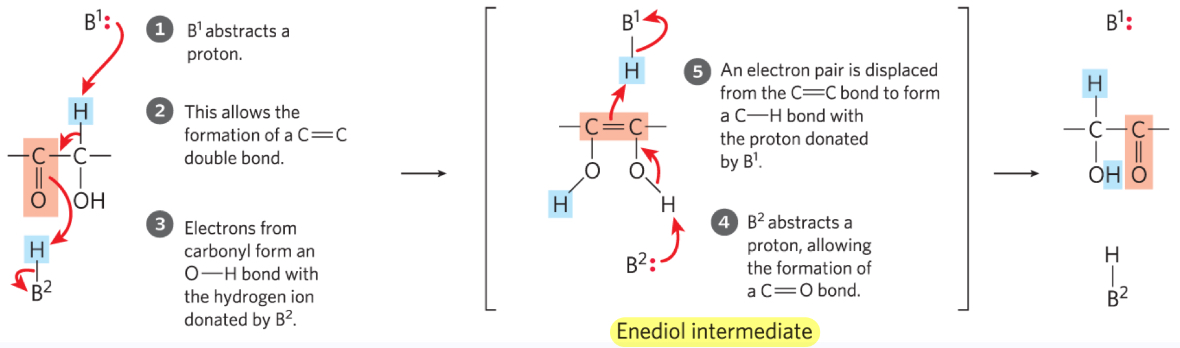
What are the 5 group transfer reactions we are studying?
Proton transfer, very common
Methyl transfer, various biosynthesis
Acyl transfer, biosynthesis of fatty acids
Glycosyl transfer, attachment of sugars
Phosphoryl transfer, to activate metabolites & signal transduction
Hydrolysis of phosphoryl group leads to release of what?
Energy
What are kinases?
Enzymes that catalyze phosphoryl group transfers WITH ATP as a donor
What are phosphorylases?
Enzyme that adds an inorganic phosphate (Pi) to the substrate. (No ATP as donor)
What are phosphatases?
Enzymes that catalyze the removal of a phosphoryl group from a phosphate ester (dephosphorylation reactions)
What are synthases?
Enzymes that catalyze condensation reactions in which NO nucleotide triphosphate (NTP) is required
What are synthetases?
Enzymes that catalyze condensation reactions that require a nucleotide triphosphate (NTP).
What are ligases?
Enzymes that catalyze condensation reactions in which two atoms are joined using ATP or another energy source
What are lyases?
Enzymes that catalyze cleavages or additions in which electronic rearrangements occur
When the nucleophilic oxygen of glucose attacks the gamma phosphoryl group on ATP what type of transfer occurs?
Phosphoryl transfer
When the nucleophilic oxygen of glucose attacks the beta phosphoryl group on ATP what type of transfer occurs?
Pyrophosphoryl transfer