chemical bonds, molecules, and ionic compounds
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
what are compounds
a substance formed when two or more different chemical elements are chemically bonded together in a fixed ratio
-ex. H2O, NaCI+, CH4
what are the 2 basic types of compounds
-molecular compounds
-ionic compounds
molecular compounds
when atoms share electrons through covalent bonds
covalent bonds
bonds made by sharing electrons
-results in the formation of molecules
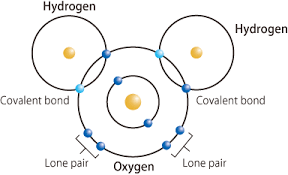
sharing of valence electrons
can be shared unequally or equally
-covalent bonds can exist as single double or triple bonds
non - polar covalent bonds
equal sharing of electrons
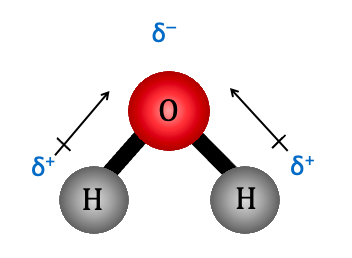
polar-covalent bonds
unequal sharing of electrons between atoms
-forms when an electronegative element is present in a molecule
what is an electronegative element
an element that attracts electrons to itself, it leads to unequal sharing of e-
ex. hydrogen & nitrogen
partial charge
exist between polar covalent bonds
-happens because one side is partially negative and the other partially positive
partiality positive
That atom has fewer electrons nearby most of the time, so it’s slightly positive
partially negative
That atom has electrons closer to it most of the time, so it’s slightly negative compared to the rest of the molecule.
what is the net charge of a polar covalent bond
net charge is still neutral because it is not actually gaining or losing electrons, they are just being shared
polar molecules
-have polar covalent bonds
-hydrophillic
-water soluble
-has an electronegative atom
non polar molecules
-have non polar covalent bonds
-hydrophobic
-water insoluble
atoms with what #’s of valence electrons will share electrons/form covalent bonds, to become stable
4,5,6
atoms with what #’s of valance electrons will lose or gain electrons/form ionic bonds, to become stable
1,2,3,7
what is an ionic compound
product of an ionic bond
-not nuetral
what happens in an ionic bonds
when one atom gives electrons to another atom so both their valance shells are stable
cation
atom that donates an electron; becomes partially charged (gained aura)
anion
electron that receives an electron; becomes negatively charged (its in debt)
ionic bonds and charges
bonds made by charge attractions, cation is attracted to anion
-one atom gives an electron to another so they both have filled valence shells
-ex. Na+CI-
ionic compounds and water
ionic compounds disassociate in water
what happens to when an ionic compound mixes with water
-negative side of water is attracted to the cation (+), positive side of water is attracted to anion (-)
-this causes the water to form hydration spheres around ions keeping them sperated
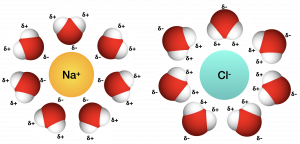
hydrogen bonds
A hydrogen bond is a weak “magnet-like” attraction between molecules.
One molecule has a hydrogen that’s a little positive.
Another molecule has something like oxygen or nitrogen that’s a little negative.
They stick together weakly — like Velcro.
what do hydrogen bonds form between
-water molecules (H&O atoms)
-amino acids
how are hydrogen bonds related to DNA
bases of dna are bonded using hydrogen bonds