Chemistry - Unit 2 Test
1/18
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
As energy increases the wavelength becomes ________________
shorter
Less energy = _________ wavelength
longer
Low Frequency = _____ energy
Low
Red =
Long wavelength
Low Energy
Low Frequency
Purple/Violet
Shorter Wavelength
High Frequency
High Energy
Coulubmic Attraction
the attraction between oppositely charged particles —> attraction between nucleus and election
Coulubmic Attraction: smaller atom means
nucleus has more charge, meaning the electrons will be pulled in more:
More Coulubmic attraction
Distance from rings to the nucleus will be less
Any with more ______ has a stronger attractive force
protons
ionization energy:
energy it takes to remove an electron (most loosely held one)
ionization energy: larger the radius=
easier to remove the electron
electronegativity
the measure of the ability of an atom’s nucleus to attract electrons from a different atom covalent bond.
What trend is this?
ionization Energy/ElectroNegativity
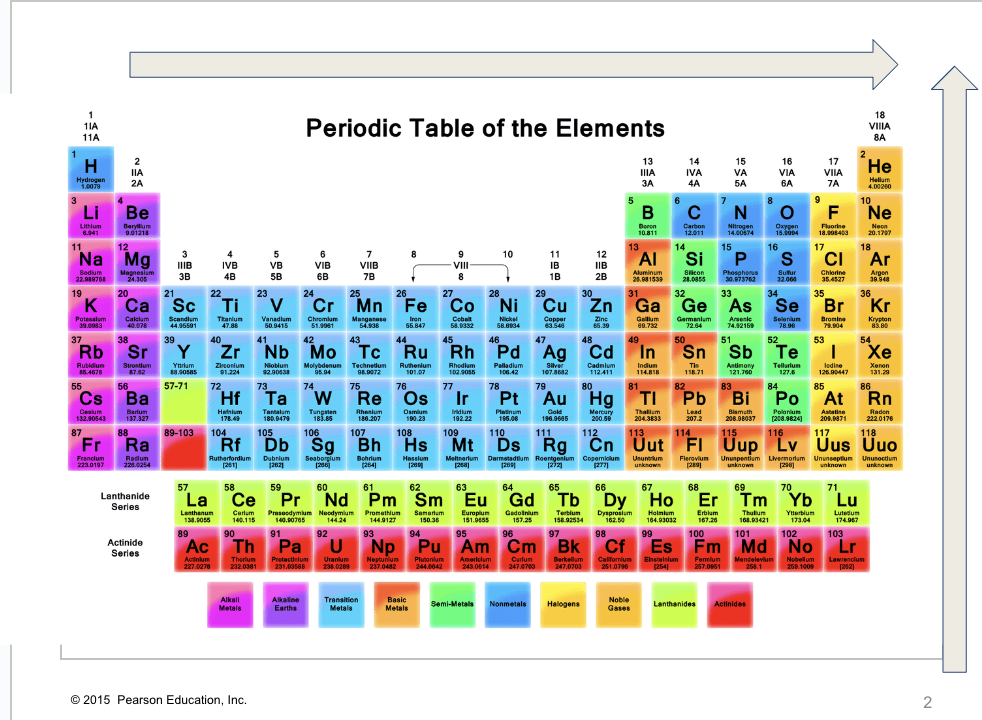
What trend is this?
Atomic Radius
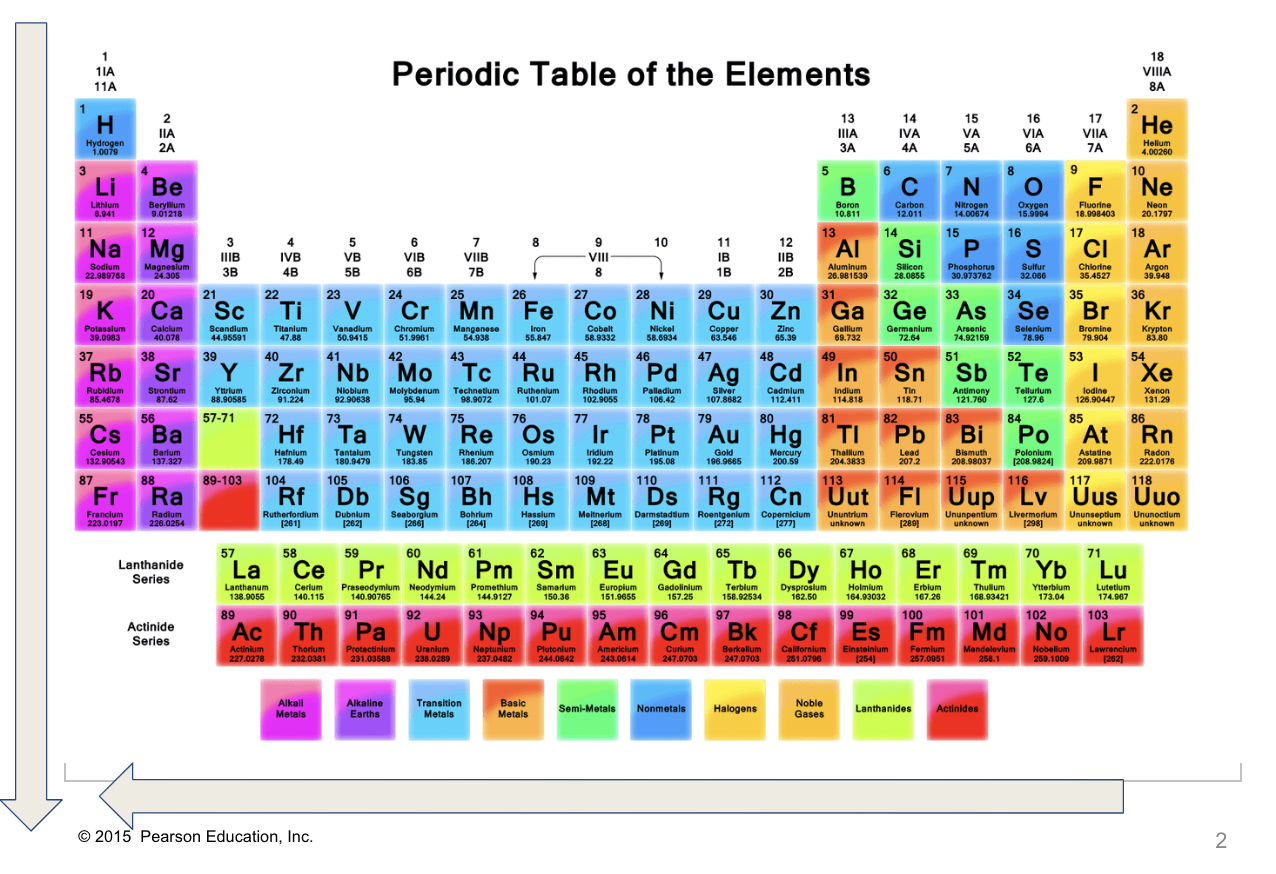
What trend is this?
Columbic Attraction
the color of emitted light is depended on the _______ of the light
wavelength
how do electrons become excited
they absorb energy
when arrows go up the energy of the electrons get ______
absorbed
when arrows go down the energy of the electrons gets ______
emitted into light