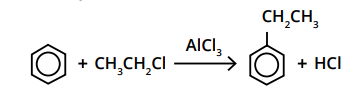Benzene
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Describe the structure of benzene.
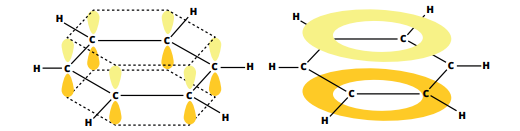
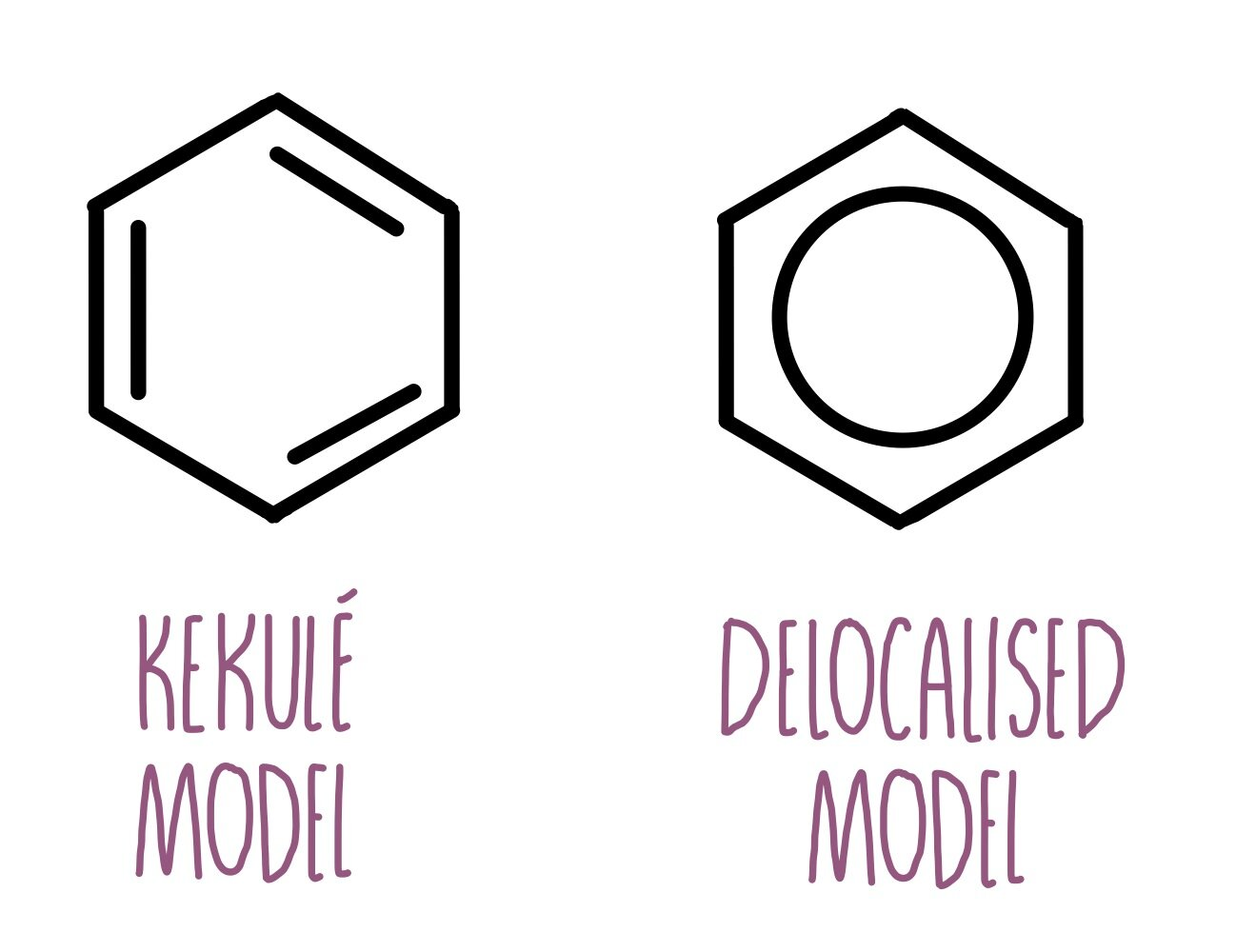
Benzene is a cyclic, planar molecule, consisting of a ring of 6 carbon atoms with 6 hydrogen atoms and a ring of delocalised electrons.
Each carbon atom is bonded to 2 other carbon atoms and a hydrogen atom by sigma bonds. The fourth valence shell electron is in a p-orbital, above and below the plane of the carbon ring.
These p orbitals overlap, forming a pi cloud above and below the plane of the ring.
This delocalised electron structure makes benzene very stable.
Each carbon-carbon bond in the benzene ring has an intermediate length between that of a double and single bond.
What is the molecular formula of benzene?
C6H6.
Briefly describe Kekule’s model of benzene.
Kekule suggested that it was a six-membered ring, containing alternating single and double bonds.
This structure is named 1,3,5-cyclohexatriene.
What 3 pieces of evidence was used to prove Kekule’s model to be incorrect?
Benzene does not undergo addition reactions (unlike compounds with double bonds) with e.g. bromine water.
Each C-C bond in benzene is equal in length.
The enthalpy of hydrogenation is less than expected. If it contained three double bonds, it would be 3 × –120 kJ mol–1, but it is in fact –208 kJ mol–1.
What does the theoretical vs experimental enthalpy of hydrogenation tells us about benzene?
If benzene contained 3 double bonds, the enthalpy of hydrogenation would be 3 x -120 = -360 kJ mol-1. This is the theoretical value.
However, the actual experimental value of the enthalpy of hydrogenation of benzene is -208 kJ mol-1.
This difference of 152 kJ mol-1 is called the resonance energy and suggests that benzene is much more stable that theorised.
What are arenes?
Compounds which contain benzene as part of their structure.
What are some properties of arenes?
High melting point- due to the high stability of the delocalised ring.
Low solubility- non-polar.
What type of reaction does benzene undergo?
Electrophilic substitution.
Why is benzene able to undergo electrophilic substitution?
Benzene has a delocalised ring of electrons which exists above and below the plane of the carbon atoms.
This is an area of high electron density, making it susceptible to attack from electrophiles.
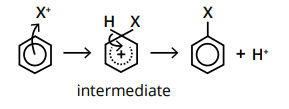
What is an electrophile?
A species which is attracted to electrons.
What are the 3 reactions of benzene to know about?
Halogenation
Nitration
Friedel-Crafts alkylation
Describe a halogenation reaction of benzene.
A halide ion with a +1 charge acts as an electrophile, attacking the electron ring.
A halogen carrier catalyst is used to generate the positively charged halide ion.
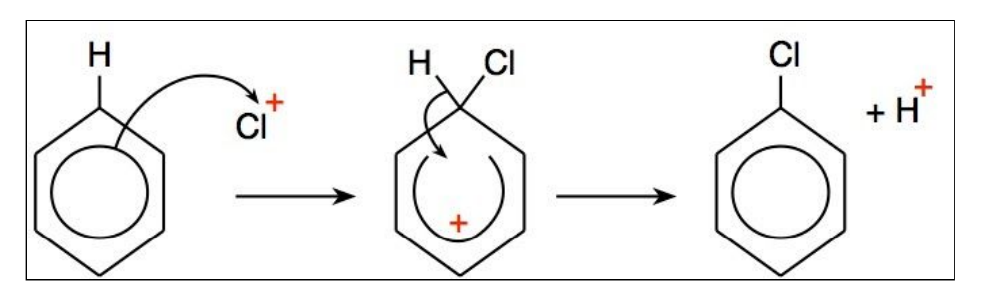
What are the conditions and reagents needed for the halogenation of benzene?
Conditions:
Room temperature
No UV light (to prevent the formation of free radicals)
Reagents:
Cl2 / Br2
Halogen carrier catalyst- AlCl3 / FeBr3
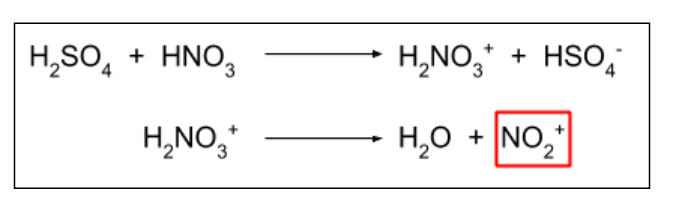
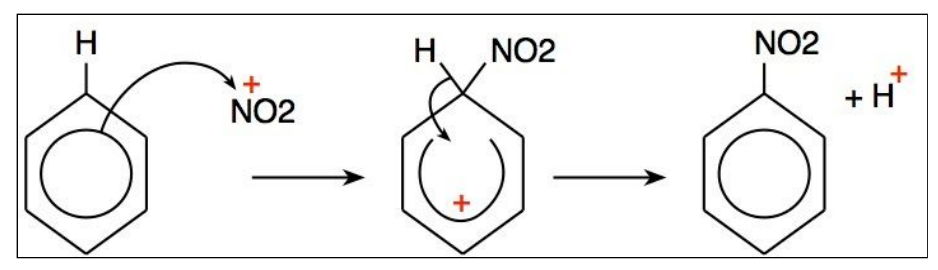
Describe the nitration reaction of benzene.
In this form of electrophilic substitution, the electrophile is NO2 + ion. This is a reactive intermediate, produced in the reaction of concentrated sulfuric acid (H2SO4) with concentrated nitric acid (HNO3).
NO2 is substituted and a hydrogen ion is released. This H+ ion reacts with the HSO4- ion from the first reaction, producing sulfuric acid. This shows how sulfuric acid is a catalyst for this reaction, since it does not get used up / is regenerated.
Draw the mechanism showing the nitration of benzene.
State the conditions and reagents needed for the nitration of benzene.
Conditions:
Temperature of 55°C
Reagents:
Concentrated sulfuric acid
Concentrated nitric acid
What happens if a temperature of greater than 55°C is used during a nitration reaction?
Multiple substitutions occur.
What is a Friedel-Crafts alkylation reaction involve?
The reaction is similar to halogenation but uses a halogenoalkane instead of a halogen, e.g. CH3Cl.
A halogen carrier catalyst is needed for this reaction to occur i.e. AlCl3.