Nucleophilic Carbon Reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Bases that can deprotonate Terminal Alkynes
NaNH2
BuLi
Mercury Catalyzed Hydration of Alkynes
Uses HgSO4 as catalyst
forms mercury bridge, adds OH makes an enol
will tautomerize to ketone (acid or base catalyzed)
Partial Hydrogenation of Alkynes
forms alkenes
Lindlar’s: forms Z-alkene
Dissolving Metal (Na/NH3): forms E-alkene
Wittig Reaction
Making:
RX + PPh3 → RPPh3
RPPh3 + BuLi (or other ba → Wittig Reagent (deprotonated RPPh3)
Reaction:
makes alkenes
negative carbon attacks carbonyl
O and P join and remove, leaving double bond
forms Z-alkenes usually between carbonyl carbon and negative carbon
Gilman Reagents
R + Li → RLi; then 2 RLi + CuCl → R2CuLi
Forms R2CuLi, adds 1 R but weaker than Grignard
Better for substitution with alkyl halides, and doesn’t fully alcoholize acid derivatives
Enolate and Enol Halogenation
Enolate (basic) - keeps adding to alpha carbon
Enol (acidic) - will add one to alpha carbon
Aldol Reactions
forms B-hydroxycarbonyl
Aldol Addition:
form enol or enolate
a-Carbon attacks other carbonyl
oxygen protonates forming B-hydroxy group
Aldol Condensation:
if a-Hydrogen present
hydrogen removed to form new enolate
E1Cb swing to remove water and form a-B double bond (enone)
will form Major Product: Z-alkene
(or if acid, water protonates then leaves)
Cross-Condensation:
uses one non-enolizable (no alpha-H) in excess
and the other added slowly
Intramoecular
Product depends on structure
ideal formation: 5-6 member ring
any less and strain prevents formation
Claisen Condensation
like aldol, but uses esters (either + other ester or carbonyl)
forms B-ketoester
base used is salt of alcohol kicked off at the end
note: B-ketoester has acidic a-Hydrogen, and so nucleophilic a-C, which can be alkylated with alkyl halogen, then hydrolyzed to form B-ketoacid, which can be decarbed to form ketone
adds ester, kicks off the alcohol, leaves with original ester with ketone at beta
note: base deprotonates the alpha-carbon to drive reaction forward, must be deprotonated later
Mixed Claisen Condensation:
one must be non-enolizable (no alpha-H), and in excess
other must be added slowly
Kinetic vs Thermodynamic Product
Thermodynamic: Zaitsev (more thermally stable)
Kinetic: Hoffman’s (less steric hinderance)
Low Temp and Strong Base favors Kinetic
Hi Temp and Weak Base favors Thermodynamic
Enone Reactions
product of Aldol Condensation
Carbonyl is electrophilic, but Beta carbon also electrophilic (1,4-nucleophilic addition/conjugate addition); will add to the 4-position (oyxgen is 1)
Carbonyl attack is kinetic
Conjugate attack is thermodynamic
Michael Addition
reacts with an enone
instead of attacking carbonyl (aldol), will attack beta-carbon (conjugate addition)
adds enol at the 4-position (alpha is 1)
will tautomerize into ketone
Note: can then undergo intramolecular aldol forming a 6-membered ring (called Robinson’s Annulation)
Dieckmann Condensation
intramolecular Claisen Condensation
also forms rings
Preparation of Enolates
can use base to deprotonate: Lithium Diisopropylamide (LDA)
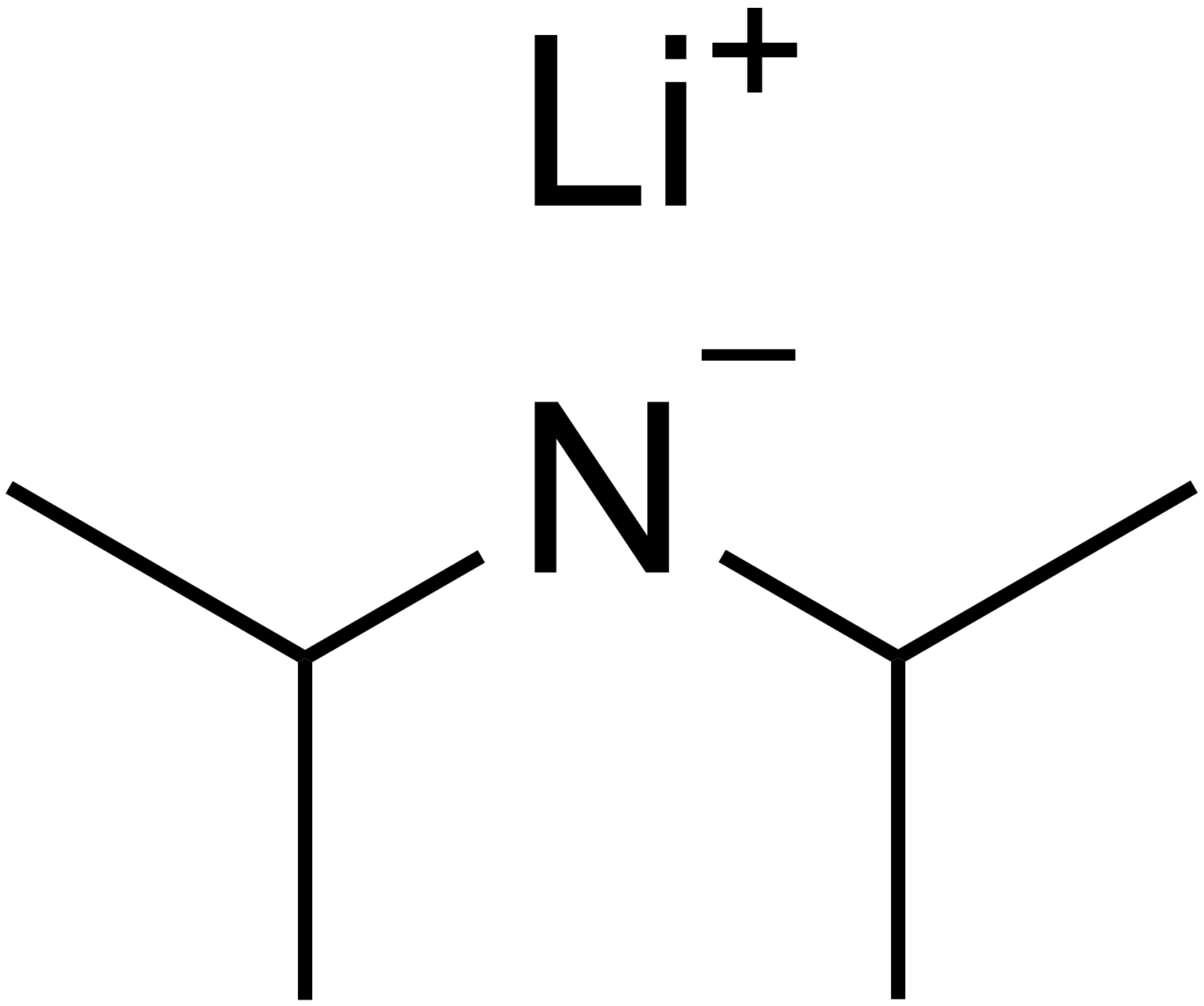
Consequences of Enolization
Exchange with Deuterium during process
Flipping of Stereochemistry during process
Racemization is a result of Number 2
a-Halogenation
addition of X to a-Carbon
Using Acid:
forms enol, double bond will add 1 Halogen
Using Base:
forms enolate, will use up all alpha-Hs to replace with Halogen
a-Alkylation
Aldehydes and Ketones:
form enolate with LDA
enolate Nu can attack alkyl halides via SN2 (base catalyzed)
Complications:
feasible with only primary haloalkanes
enolate may undergo Aldol instead
Ketones may undergo multiple alkylation or regioisomeric products
As such, ideal conditions are only one R group attaches so only one alpha-H available
Enamines:
more stable than enol (will not tautomerize as much)
nucleophilic even if neutral (base less needed)
enamine can be hydrolyzed after reaction to return to ketone
as such, enamine formation, addition, hydrolysis is a valid pathway (carbonyl + secondary amine)
Does not work with alcohols (will just take proton in acid base)
Acetoacetic Ester Synthesis
starts with B-ketoester (ex: ethyl acetoacetate)
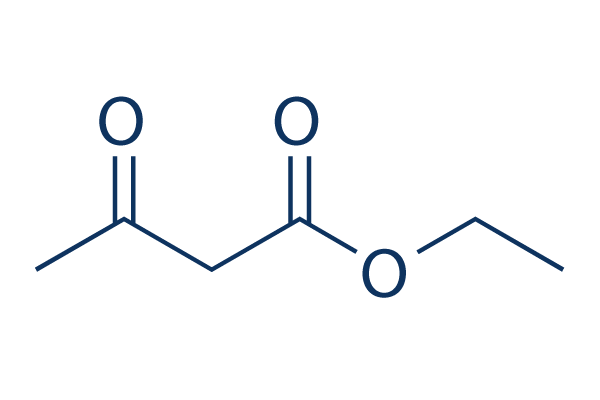
forms enolate, then reacts it with alkyl halides (can add 1 R group, or can be looped to add 2 R groups)
then treat with acid to hydrolyze ester into carboxylic acid
heat will decarboxylate leaving behind a ketone
Useful for forming substituted ketones from B-ketoesters
Malonic Ester Synthesis
uses a dibeta-ester
form enolate, react with alkyl halide (can be looped to add 2 R Groups)
acidify to convert both groups to carboxylic acids
heat to decarboxylate one of the groups
left with substituted carboxylic acid
almost exact same reaction as Acetoacetic Ester Synthesis