neutralisation (titration)
1/3
Earn XP
Description and Tags
Neutralisation reactions occur when an acid reacts with an alkali. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). In this practical, you will perform a titration to find the volume of sulfuric acid that is required to neutralise 25 cm3 of sodium hydroxide solution. You will then use the volume of sulfuric acid required to calculate the concentration of the sulfuric acid.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
aim
determine the reacting volume of solutions of a strong acid and a strong alkali by titration
equipment
sodium hydroxide solution (with known concentration)
sulphuric acid solution (unknown concentration)
burette
clamp and stand
funnel
bulb pipette & safety fillers
indicator
setup
measure out an exact volume of sodium hydroxide solution with a bulb pipette
attach safety fillers to top of pipette and place it in solution - suck up solution into pipette until the bottom of the meniscus reaches the 25cm³ line
drop solution into conical flask
pour sulphuric acid into burette using a funnel until the bottom of the meniscus reaches the 0 line
place conical flask underneath the burette
add some pH indicator e.g. methyl orange into acid (where it appears yellow)
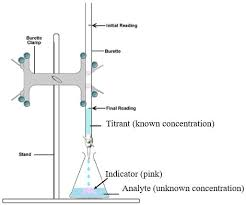
steps
first, do a rough titration to know around how much acid needs to be added
open the tap and swirl the flask
when colour change stops occurring stop titration
repeat, use rough titration to estimate when to add smaller volumes of acid for accuracy
when colour change occurs, record final volume of acid in burette
repeat until achieve concurrent results (results in 0.1cm³) and calculate the mean
can then calculate concentration of unknown solution