Lectures 7 & 8 - Intro to brain - glutamate & GABA
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What is glutamate
Main excitatory transmitter in CNS
synthesised from glutamine
activate large family of ionotropic and metabotropic receptor
what is GABA
Main inhibitory transmitter
synthesized from glutamate
glycine (amino acid transmitter)
activates a large family of inotropic and metabotropic receptors
Glutamate
amino acid aspartate

GABA
Amino acid glycine

Where is glutamate found
Pyramidal neurones
neurones shaped like a pyramid
Where is GABA found
interneurons (shorter local)
projection neurones
Where is glutamate synthesised from
glucose in krebs cycle
or
glutamine in gilal cells
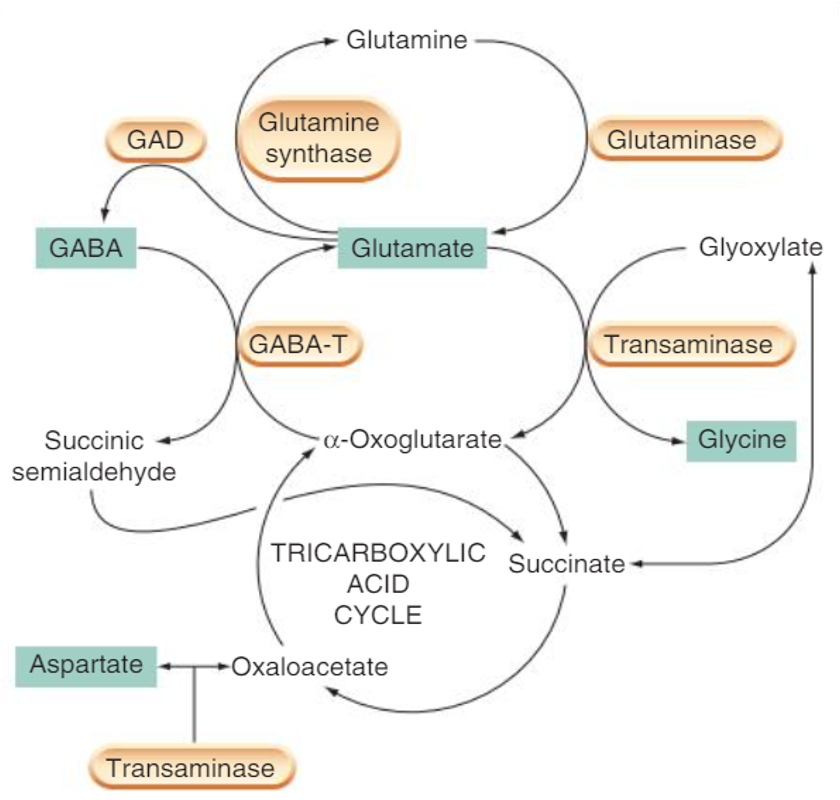
How is glutamate converted
contains GAD which converts glutamate to GABA
•Glutamate can be converted to GABA by the enzyme glutamic acid decarboxylase – GAD
How is glutamate metabolisd
stored in synaptic vesicles inside neurons, and released into the synapse when calcium signals trigger exocytosis.
Once released, excess Glu is quickly cleared by EAAT transporters, which move it into neurons and astrocytes (supporting glial cells).
In astrocytes, Glu is converted into glutamine (Gln) by the enzyme glutamine synthase.
Glutamine (Gln) is transported back into neurons by GlnT transporters.
In the neuron, glutaminase converts glutamine back into glutamate, ready to be used again.
Before release, glutamate is packaged into vesicles by Vesicular Glutamate Transporters (VGlutT).
How is GABA metabolised
Start with glucose (energy source):
Glucose enters the TCA cycle (Krebs cycle).
Through glycolysis and acetyl-CoA, it produces α-ketoglutarate, a key intermediate.
Formation of Glutamate:
α-ketoglutarate is converted into glutamate (an excitatory neurotransmitter).
Synthesis of GABA (inhibitory neurotransmitter):
Glutamic acid decarboxylase (GAD) converts glutamate → GABA.
This is the main pathway of GABA production.
Breakdown of GABA:
GABA is broken down by the enzyme GABA transaminase into succinic semialdehyde.
(💡 Drugs that inhibit GABA transaminase, like vigabatrin, increase GABA levels and are used in epilepsy treatment.)
Entry back into TCA cycle:
Succinic semialdehyde is converted into succinate by succinic dehydrogenase.
Succinate re-enters the TCA cycle, closing the loop.
Describe the function and structure of ionotropic receptors
•Multisubunit receptors
•Heterogeneous receptors (not same subunit)
•Affects physiological function and pharmacology
•Rapid cellular effects
aka LIGAND GATED ION CHANNELS
Describe the function and structure of metabotropic receptors
•Hetero- and homodimers
•Activate second messenger systems
• effects on synaptic transmission
•May be “autoreceptors” located presynaptically on nerve terminals
aka g-protein coupled
Describe GABA A
Chloride ion channel
inhibitory
Gaba B
7 transmembrane protein coupled to trimeric g alpha, g beta and g gama protein
inhibitory
Gaba C
chloride ion channel
inhibitory
Describe the structure of ionotropic GABA receptors
pentameric
6 different alpha
3 beta
3 gamma
1 gamma
1 e
3 P
ligang gated
fast hyperpolarisation→ inhibition
Where are the main sits of drug action on GABA a receptors
Orthosteric : receptor site e.g. gaba agonists, channel blocking drugs
Allosteric site: modulatory site: benzodiazipine agonists, antagonists, benzodiazepine inverse agonists, channel modulators
how do gaba receptors induce an inhbitory effects
chloride ions enter the channel
create more negative
moves action potential away from threshold
What anesthetics are GABA
e.g. etomidate & propofol are volatile anaesthetics that act at GABAARswhat
are barbituates
e.g. pentobarbital has sedative/anticonvulsant effectswh
what are benzodiazpine
Multiple actions e.g. anxiety relieving, anticonvulsant, hypnotic
what are neurosteroids
•Neurosteroids -Metabolites of progesterone & deoxycorticosterone
Describe how is gaba heterodimer
contains R1 and R2
closes ca2+ channels presynaptically to reduce transmitter release
autoreceptor
Open potassium channels postsynaptically eliciting a slow hyperpolarization
spacisity
rigidity in muscles and limbs
Iono glutamate receptors
tetrameric
NMDA: 7 subunits
AMPA:
KAINATE:
How do glutamate receptors induce excitatory response
Sodium enters channel pore
creates more positivity
drives action potential closer to threshold
excitatory postsynaptic potential
fast excitatory neurotransmission
how do NMDA receptors work
cell depolarised
Mg2+ block is removed
activation requires glycine and glutamate
examples: ketamine, memantine
metabotropic glutamate receptors
8 different G-protein coupled receptors
group 1, group 11, group 111
slow neuromodulatory role
no drugs on the market
glutamate presynaptically
increases glutamate release through NMDAr
Presynaptic mGluR decreases glutamate release by decreasing ca2+
how does glutamate bind to so many receptors
its not a rigid molecle rotates about aB and By bonds
has different conformations
nine rotamers