Exam 1
5.0(1)
Card Sorting
1/61
Earn XP
Description and Tags
chaps. 1-3
Last updated 12:01 AM on 9/10/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
1
New cards
If an atom makes 2 double bond or 1 triple bond then the hybridization must be
sp
2
New cards
If an atom makes no multiple bonds then the hybridization must be
sp3
3
New cards
If an atom makes one double bond then the hybridization must be
sp2
4
New cards
What does ARIO stand for and when do you use ARIO?
stands for Atom, Resonance, Induction, and Orbital; use to find which way the equilibrium is shifted, the more stable the base the weaker it is and equilibrium shifts towards the weaker acid
5
New cards
When deciding which acid is stronger, if the elements are in the same ROW, then determine which element has more
electronegativity, more eletronegtivity means more acidic proton
6
New cards
When deciding which acid is stronger, if the elements are in the same GROUP (column), then determine which element has
the bigger size; as size increases anion stability increases
7
New cards
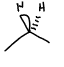
What functional group is this?
Alkane
8
New cards
What is the PH of alkane?
about 50
9
New cards
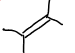
What functional group is this?
Alkene
10
New cards
What is the PH of alkene?
about 40
11
New cards
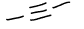
What functional group is this?
alkyne
12
New cards
What is the PH for alkyne?
about 25
13
New cards
What is the PH for alcohol?
about 16
14
New cards
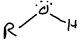
What is this functional group?
alcohol
15
New cards
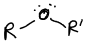
What is this functional group?
Ether
16
New cards
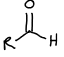
What is this functional group?
Aldehyde
17
New cards
What is the PH of aldehyde?
not acidic
18
New cards
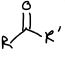
What is this functional group?
Ketone
19
New cards
What is the PH of carboxylic acid?
about 5
20
New cards
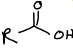
What is this functional group?
carboxylic acid
21
New cards
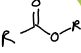
What is this functional group?
Ester
22
New cards
What is the PH of amide?
about 30
23
New cards
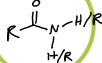
What is this functional group?
amide
24
New cards
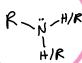
What is this functional group?
amine
25
New cards
What is the PH of thiol?
about 8
26
New cards
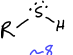
What is this functional group?
Thiol
27
New cards
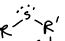
What is this functional group?
sulfide
28
New cards
What is the equation for formal charge?
valence electrons - number of bonds - 2(lone pairs)
29
New cards
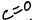
What is this functional group?
carboynl
30
New cards
What is the most electronegative element?
Florine
31
New cards
If the electronegativity is less than 0.5 the molecule is
nonpolar
32
New cards
If the electronegativity is between 0.5-1.7 the molecule is
polar
33
New cards
If the electronegativity is greater than 1.7 the molecule is
ionic
34
New cards
What is the shape for sp3 orbitals?
tetrahedral
35
New cards
What is the bond angle for sp3 orbitals?
109\.5 degrees
36
New cards
How many sigma and pi bonds do sp3 orbitals have?
4 sigma bonds 0 pi bonds
37
New cards
What is the shape for sp2 orbitals?
trigional planer
38
New cards
What is the bond angle for sp2 orbitals?
120 degrees
39
New cards
How many sigma and pi bonds do sp2 orbitals have?
3 sigma and 1 pi bond
40
New cards
What is the shape for sp orbitals?
linear
41
New cards
What is the bond angle for sp orbitals?
180 degrees
42
New cards
How many sigma and pi bonds do sp orbitals have?
2 sigma and 2 pi bonds
43
New cards
Who donates the protons (H+) in BLAB?
the acids
44
New cards
Who receives the protons in BLAB?
the base
45
New cards
To predict equilibrium in acid/base mechanisms
favor side with weaker acid using pka
46
New cards
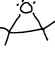
What functional group is this?
expoxide
47
New cards
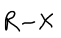
What is this functional group?
haloalkane/halogen
48
New cards
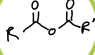
What is this functional group?
acid anhydride
49
New cards
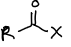
What is this functional group?
acyl halide
50
New cards
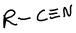
What is this functional group?
nitrile
51
New cards
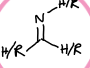
What is this functional group?
imine
52
New cards
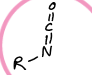
What is this functional group?
isocyanate
53
New cards
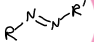
What is this functional group?
azo compound
54
New cards
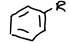
What is this functional group?
arene/aromatic
55
New cards
What is the pka for sulfuric acid?
\-9
56
New cards
What is the pka for amines?
about 35
57
New cards
What is the pka for HI, HBr, and HCl respectivly?
\-10,-9,-7
58
New cards
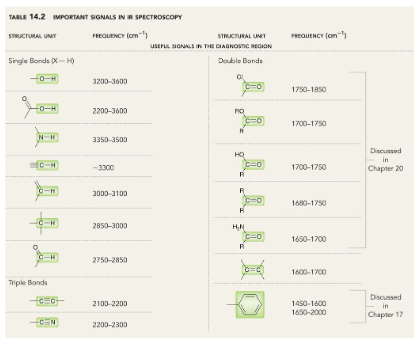
How to use IR Spectrosopy. (CHART)
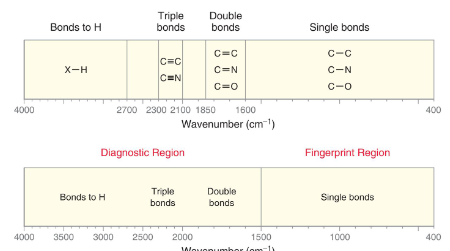
59
New cards
How many lone pairs does Florine get?
3
60
New cards
How many lone pairs do oxygen get?
2
61
New cards
How many lone pairs does Nitrogen get?
1
62
New cards
Analyzing a IR Spectrum. (CHART)