CHEM 3300 / Topic 6c: Solid-State - Defects and Electronic Conductivity
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What phase of matter contains defects?
All solids contain defects.
What three material properties do defects affect?
> Mechanical strength.
> Electrical conductivity.
> Chemical reactivity.
Why do defects occur?
Defects occur because they introduce disorder (associated withy ∆S > 0).
What are the four different types and subtypes (if any) of defects found in solids? Briefly describe each.
> Intrinsic defects: Defects occurring in a pure substance.
> Extrinsic defects: Defects occurring because impurities are present.
> Point defects: Defects occurring at single sites and are random interruptions in a lattice.
> Extended defects occurring in 1 or 2 or 3 dimensions.
What are the three types of extended defects?
> Line defects: Defects occurring in 1D.
> Shear defects: Defects occurring in 2D.
> Crystallographic defects: Defects occurring in 3D.
What are Wadsley defects?
A type of extended defect, they are crystallographic shear planes randomly distributed in the solid.
What are the three types of intrinsic defects?
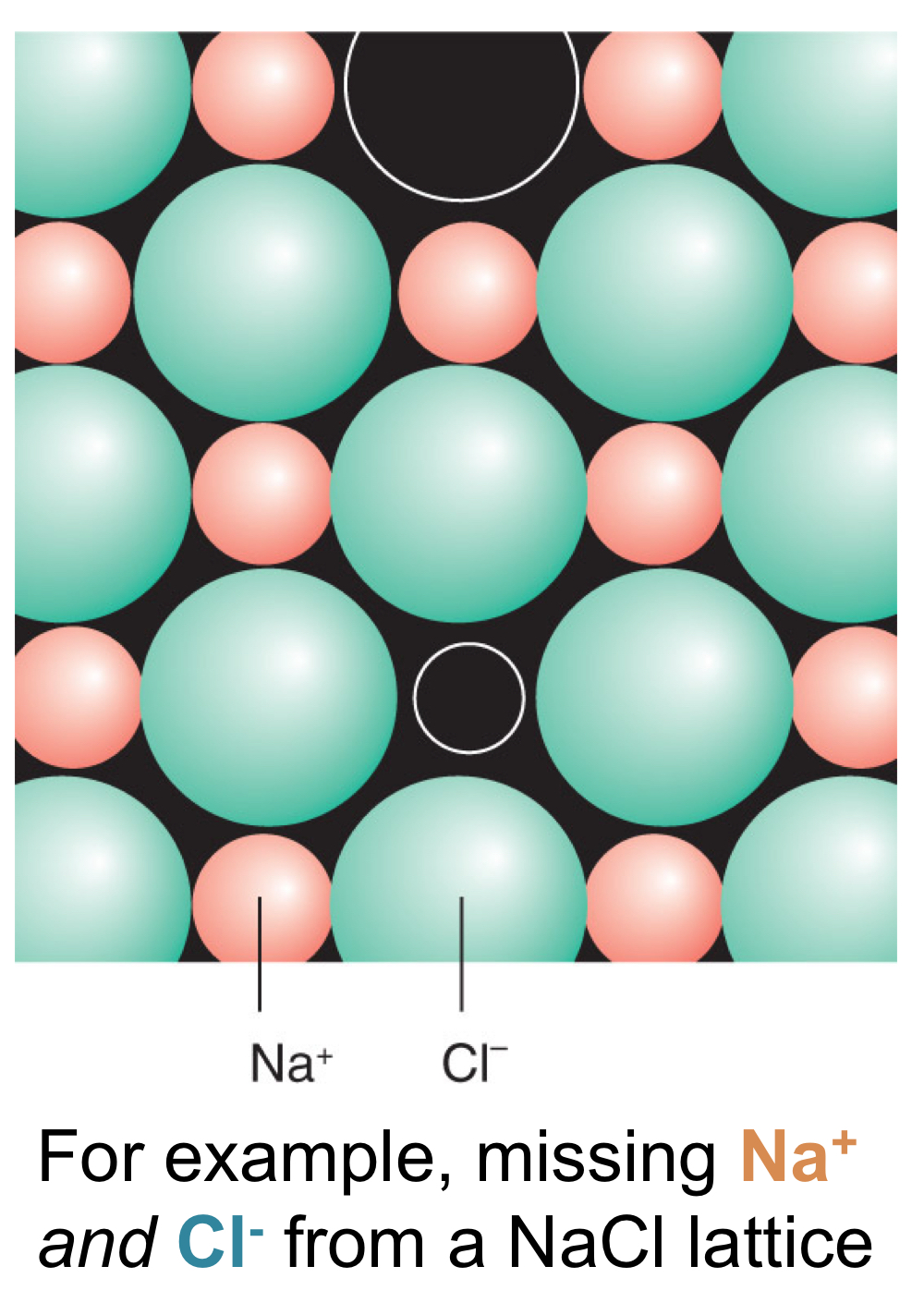
> Schottky defects: Vacancy in lattice, i.e. missing atom or ion.
> Frenkel defects: Displacement of species in lattice.
> Anti-site defect: Exchange of atoms within a lattice.
DEFECT CONCENTRATION AND ENERGIES
What is the relationship between defect concentration and the following from G = H - TS:
H
TS
G
[defect] increases, H increases linearly.
[defect] increases, T∆S increases in a curved fashion (for reasons outside the scope of this course).
[defect] increases, G decreases and then increases in a curved fashion.
DEFECT CONCENTRATION AND G
What explains the decrease and increase of G as [defect] increases?
This decrease to a finite energy level of G and subsequent increase of G is due to the fact that, as defects increase, even as you are introducing entropy, you’re also increasing so much disorder to the point that you’re actually destabilizing the structure, a.k.a. raising G.
INTRINSIC POINT DEFECTS
Where structures are Schottky defects most common? Why would these structures let Schottky defects occur?
In salts, how do Schottky defects enter the show, and why?
Where structures are Frenkel defects most common? Why would these structures let Frenkel defects occur?
Structures with high coordination numbers, due to the enthalpy penalty (of reducing the average coordination number of the remaining atoms) is relatively low.
If I had a lot of pairs of cations and anions. Like, a bazillion pairs of them. If I remove one pair of them, the whole group of pairs won’t get affected that much - the group will still be stable.
If I didn’t have a lot of pairs of cations and anions. Say I had very few pairs in the lattice. If I remove one pair, the whole group will be affected and the overall lattice will be unstable.
They occur in pairs to maintain charge neutrality.
Structures with lower coordination numbers, because they have more open space, allowing species to move around without affecting long-range order of structure.
If I was an atom in a lattice and I wanted to go to a space that was empty in the lattice, it will be more difficult for me to get to that space if there was a crowd (high coordination) than if there were a few atoms (low coordination).
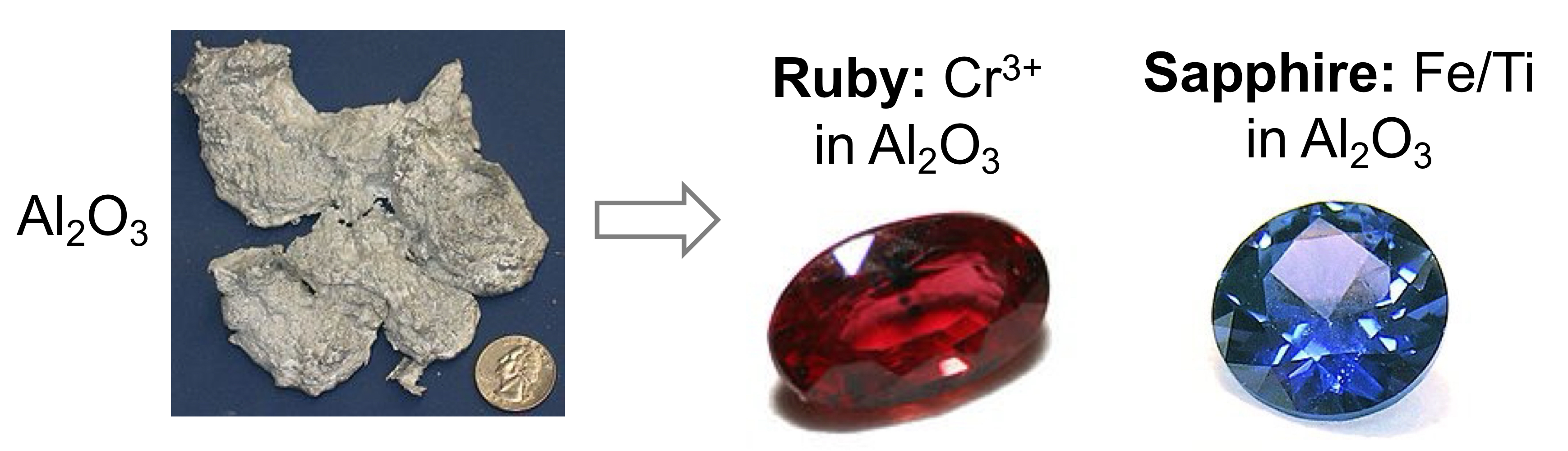
EXTRINSIC POINT DEFECTS
Where are extrinsic point defects most common, and what are they responsible for?
What are dopants?
What is the one significant requirement for a dopant to be added to a material?
Of materials, what one significant thing can a dopant modify?
Would impurities create substitional alloys? Why so?
Common in minerals, responsible for properties of gemstones.
Impurities intentionally added to material in very small amounts to leave host lattice intact.
Similar rionic to the atoms they replace.
Electronic structure of materials.
No, due to differences % of substitutional atoms between substitutional alloys and extrinsic point defects.
WADSLEY DEFECTS
What again are Wadsley defects? What category of defects do they belong to?
Belonging to extended defects, these are defects occurring in 3D with crystallographic shear planes, which are new planes produces when an plane of atoms, i.e. oxygen in WO3-x, in the original structure is removed and the rest of the structure shears to accommodate this.
SEMICONDUCTORS
What are metalloids?
Why are semiconductors called semiconductors?
What are two important intrinsic semiconductors on Earth? Why are they called as such?
What do orbitals in a solid lead to in the grand scheme of conductivity?
Solids that are not metallic enough to be considered metals and not non-metallic enough to be called non-metals.
Semiconductor conductivity is between insulators (not conductive, e.g. salts) and conductors (metals).
Silicon and Germanium, because they are semiconducting in their pure forms.
Overlap of many atomic orbitals in a solid leads to many molecular orbitals closely spaced and similar in energy and forms bands of energy levels separated by band gaps.
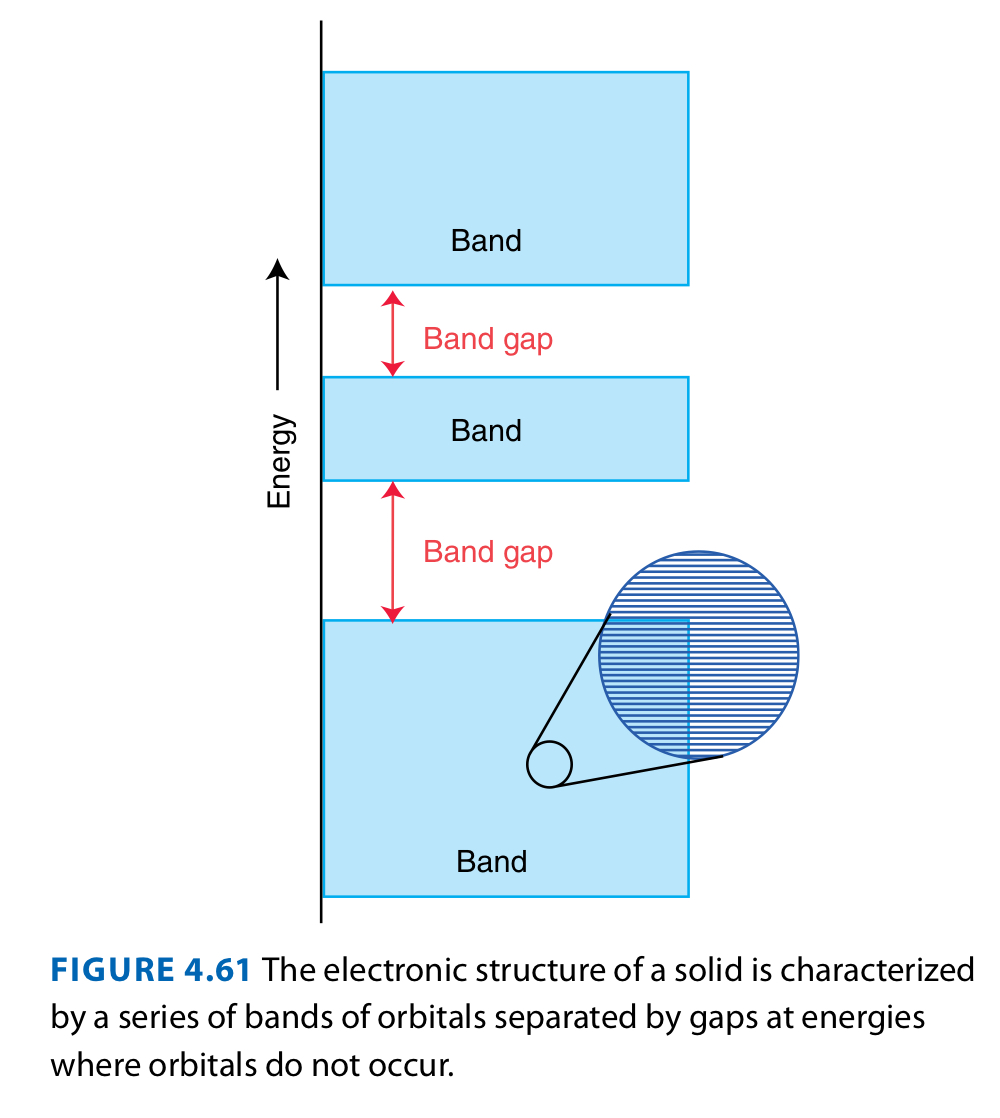
BANDS
Just to remind you, what regions on a series of bands can electrons occupy? What can they not occupy? Why so?
Just to remind you, what does each atom give to the entire electronic structure?
Bands are built from atomic orbitals with similar energy, and so we can have __, __, and __ bands.
Why are there energies molecular orbitals cannot occupy?
Bands can be occupied, because they consist of accessible energy levels. Band gaps cannot be occupied, because they don’t have energy levels that they can access.
Its valence electrons.
Bands are built from atomic orbitals with similar energy, and so we can have s, p, and d bands.
Big gap of s and p band energies.
DENSITY OF STATES
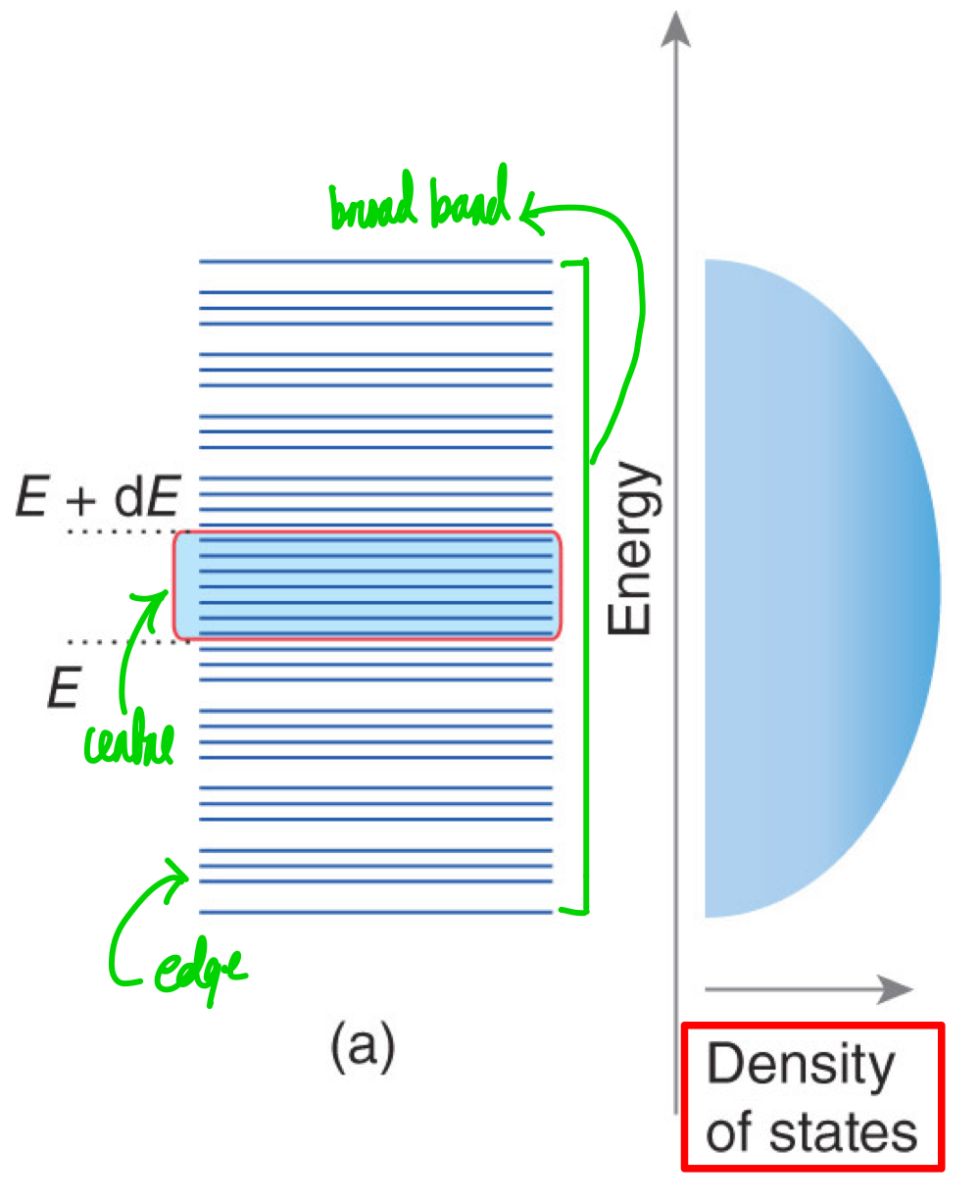
What is density of states?
Where is the density of states typically greater? How about the lowest?
What’s the relationship between number of orbitals and number of states?
In regards to number of atomic orbitals and strength overlap, what could possibly make a broad band with high density of states?
In regards to number of atomic orbitals and strength overlap, what could possibly make a narrow band with low density of states?
The number of energy levels in an energy range in the width of a range.
At the centre is the greatest. At the edges is the lowest.
Proportional.
Large numbers of contributing atomic orbitals with strong overlap produce broad bands with high density of states.
Small numbers of contributing atomic orbitals with atoms that are well-separated produce a narrow band.
FERMI LEVEL AND ELECTRONIC CONDUCTIVITY
What’s a Fermi level?
Relate the words metals, nonmetals, metalloids and the words conductors, insulators, and semiconductors.
The highest occupied orbital at T = 0K.
These are the relationships:
Metals are conductors.
Nonmetals are insulators.
Metalloids are semiconductors.
CONDUCTOR BANDS
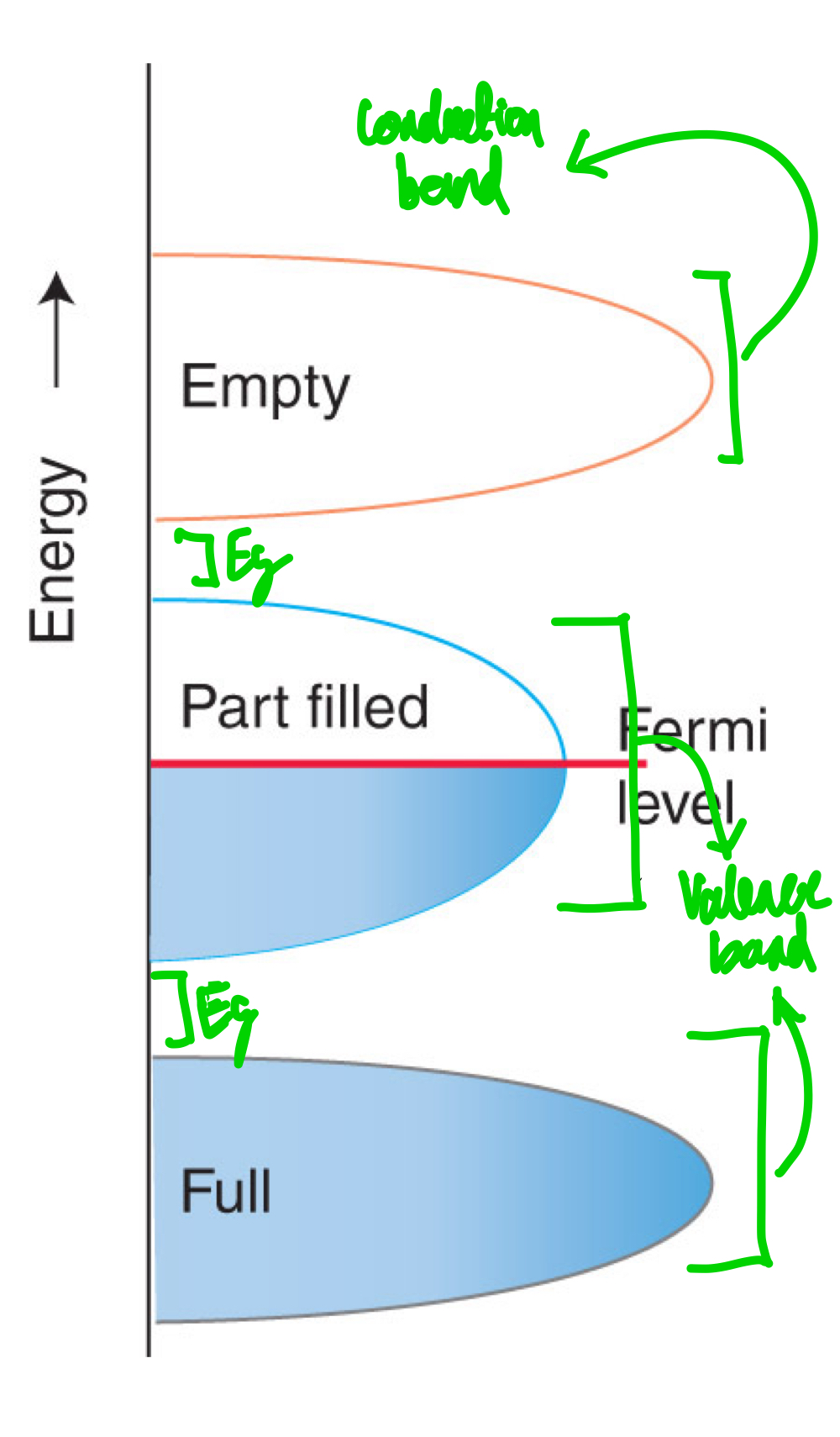
What does a band look like in conductors?
When can you consider electrical conductivity present in a metal?
In technical terms of metallic conductivity, what’s the trend we see with conductivity and temperature? Explain this trend.
In layman’s terms of metallic conductivity, what’s the trend we see with conductivity and temperature? Explain this trend.
If you can’t use thermal energy to promote electrons in conductors due to carrier scattering, what type of energy can you use to promote?
The valence band is not completely full, but electrons close to the Fermi level can be easily promoted to nearby levels.
When electrons are mobile and moving.
Conductivity decreases with increasing temperature, because the metals are already good conductors, and increasing temperature increases the vibration of species, which leads to the scattering of charge carriers.
Conductivity decreases with increasing temperature, because the metal - the conductor - has already good flow of traffic, now you’re just making cars bump into each other because of road rage.
Light energy.
INSULATOR BANDS
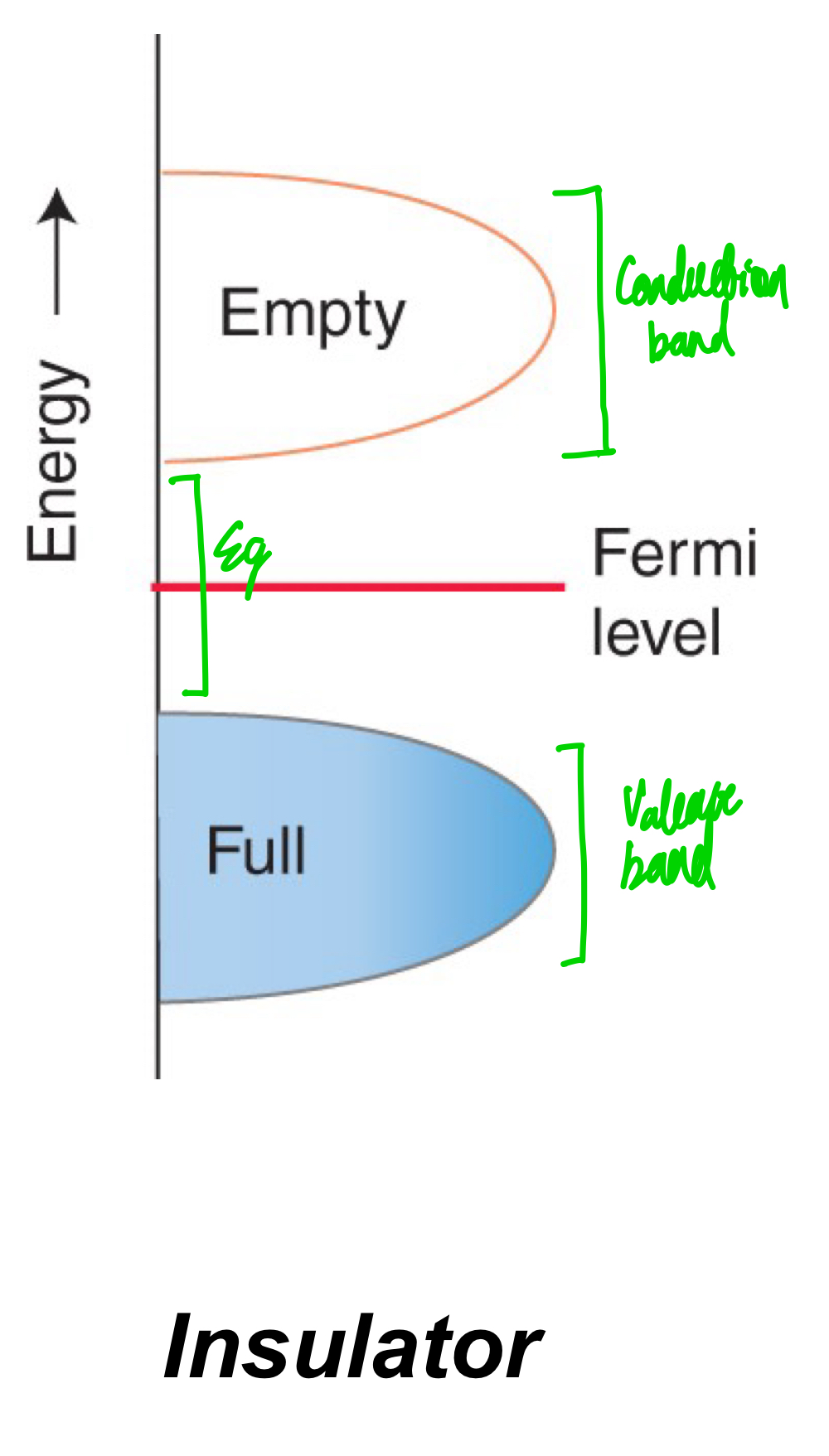
What does a band look like an insulator?
Can you consider electrical conductivity to be present in an insulator? Why so?
The valence band is completely full, and there is an energy gap that takes a lot of energy to promote an electron from the valence band to the next available empty orbital in the conduction band.
No, because the energy gap is too big for electrons to jump across to reach the conduction band.
INTRINSIC SEMICONDUCTOR BANDS
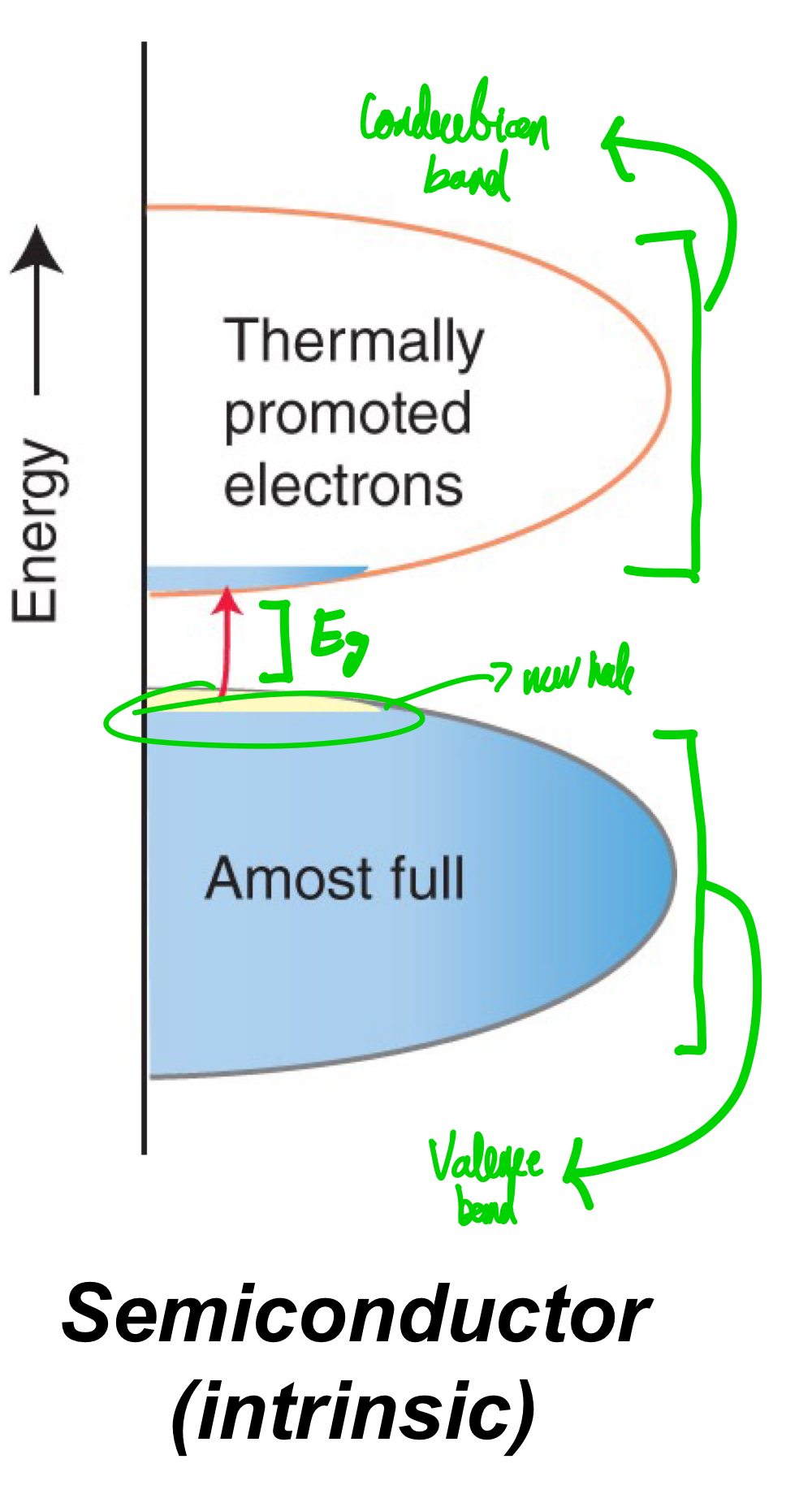
What does a band look like an intrinsic semiconductor?
When electrons are mobile in an intrinsic semiconductor, what do electrons leave behind?
Can you consider electrical conductivity to be present in an intrinsic semiconductor? Why so?
In terms of pure metalloid conductivity, what’s the trend we see with conductivity and temperature?
The valence band is full, but the band gap is small enough that thermal energy alone can lead to promotion from valence band to the conduction band.
Holes of positive charge.
Yes, because both electrons and holes are mobile.
Conductivity increases with increasing temperature as more electrons can be promoted into the conduction band.
EXTRINSIC SEMICONDUCTOR BANDS
What is an extrinsic semiconductor?
What are the two types of extrinsic semiconductors? Briefly describe each.
A semiconductor due to dopants.
The two types are:
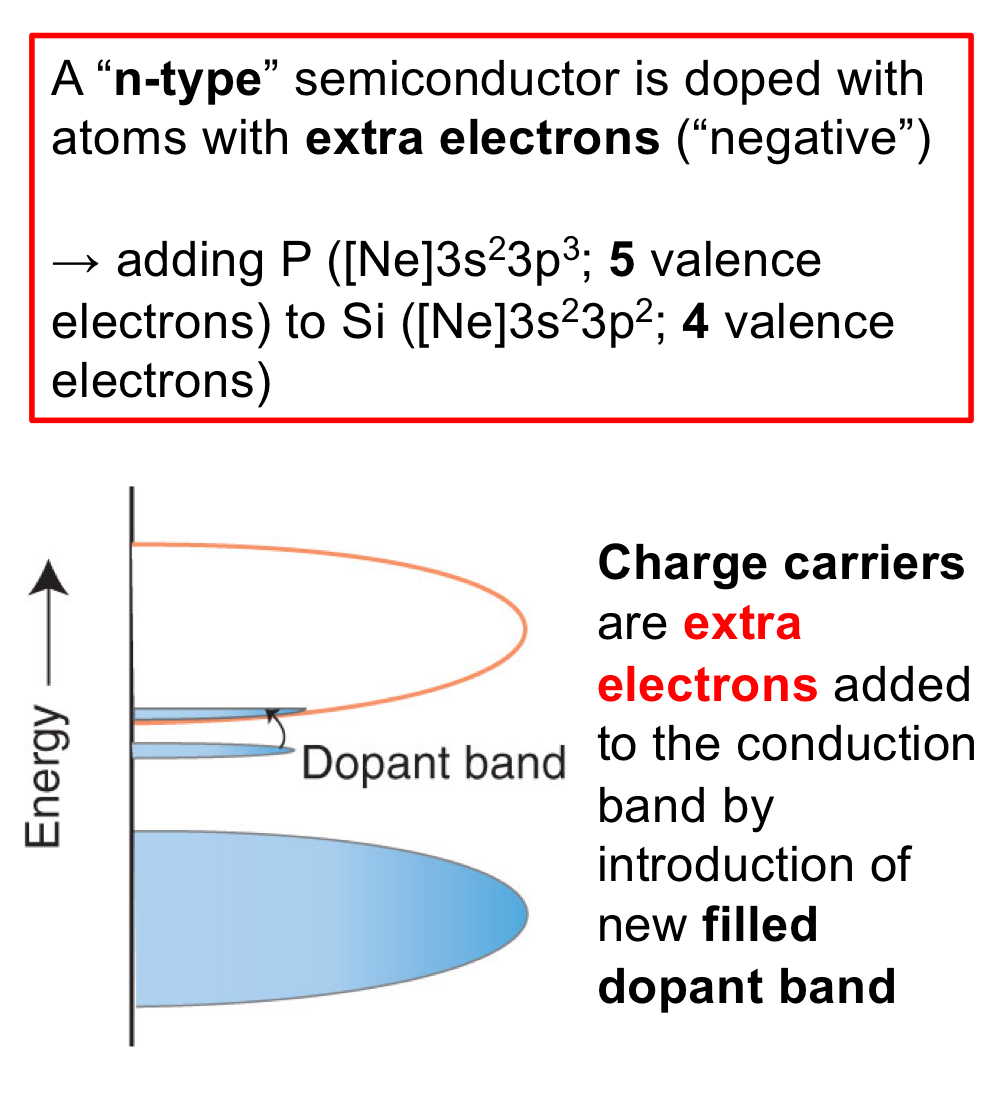
n-type semiconductor: Semiconductor doped with atoms with extra electrons, bringing negative charge.
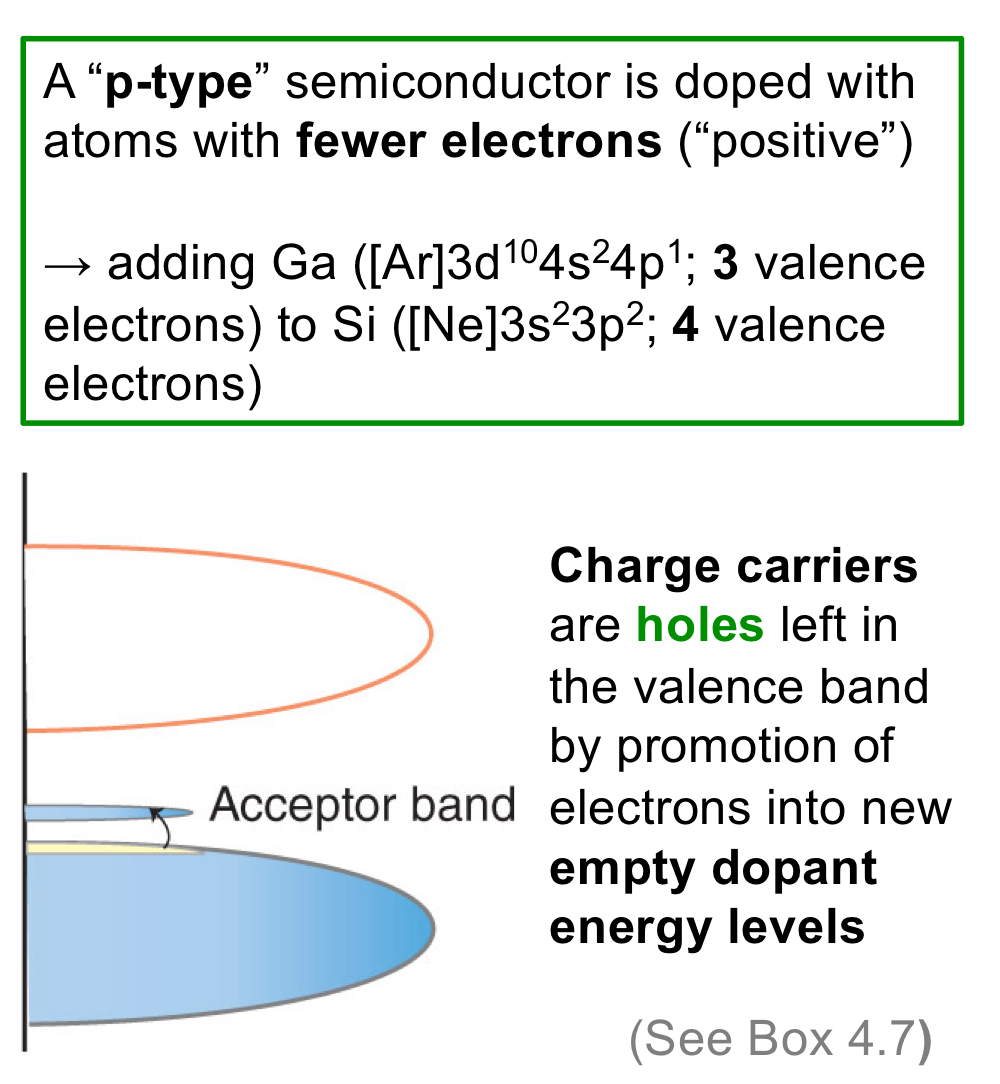
p-type semiconductor: Semiconductor doped with atoms with fewer electrons, bringing positive charge.
N-TYPE SEMICONDUCTORS
In regards to band theory, what do charge carriers do in n-type semiconductors?
The charge carriers, which are extra electrons, are added to to the conduction band by introduction of newly filled dopant band.
P-TYPE SEMICONDUCTORS
In regards to band theory, what do charge carriers do in p-type semiconductors?
The charge carriers, which are holes, have electrons promoted to them from the valence band.
MISCELLANEOUS
Why can’t you take electrons present in energy levels far away from the Fermi level? Make an analogy.
How can holes, the absence of electrons, move? Make an analogy.
Think about taking a rock from a pile of rocks. Would you rather get a rock from the outside or rock from the inside? Why waste energy of getting a rock from the inside when you can get a rock from the outside?
Imagine having a tray of a dozen eggs, wherein 10 eggs and 12 vacant holes are present. When you move an egg into a hole, you just created a new hole. The hole moved.