Silverstein and Hopper Chapter 107: Dyshemoglobinemias
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Structure of Hemoglobin
Hemoglobin is composed of four polypeptide chains (globins) each attached to a heme molecule
Heme is made up of a tetrapyrrole with a central iron molecule
Oxygen binds to the central iron molecule in the ferrous (Fe++) form
Each hemoglobin molecule carries four oxygen molecules
Pathophysiology of Carbon Monoxide Toxicity
CO is absorbed rapidly through the lungs at the level of the alveolus
Quantity of gas absorbed is dependent on minute ventilation (respiratory rate x tidal volume), duration of exposure, and concentrations of CO and oxygen in the environment
Once absorbed in the blood and circulated throughout the body, a small amount of CO is oxidized to CO2, some remains as gas in solution, and some binds to proteins including Hb, myoglobin, and cytochromes in mitochondria
What are the two main mechanisms of CO toxicity?
Impaired oxygen delivery to tissues (hypoxia via dyshemoglobinemia)
Direct cellular toxicity
Mechanisms of CO Toxicity - Impaired Oxygen Delivery to the Tissues
CO displaces oxygen from Hb and causes an allosteric hinderance of oxygen release from Hb to tissues
CO completes with oxygen for Hb bindings sites with 200-240 times the affinity
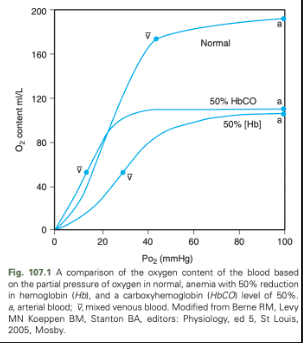
CO binds 2/4 available heme groups in each molecule of Hb, causing a decrease in oxygen carrying capacity of 50%
Low levels of CO in the blood result in markedly reduced oxygen carrying capacity despite a normal Hb concentration and normal PO2
Oxyhemoglobin dissociation curve is shifted down and to the left resulting in decreased release of oxygen to the tissues
Mechanisms of CO Toxicity - Direct Cellular Toxicity
Can be due to CO binding heme proteins other than Hb, including cytochromes, myoglobin, and guanylyl cyclase
Binding to cytochrome a3 disrupts oxidative metabolism, potentially causing generation of oxygen free radicals and impaired cellular respiration
Binding to myoglobin can cause myocardial hypoxia, depression, and arrhythmias, as well as direct skeletal muscle toxicity and rhabdomyolysis
Binding of guanylyl cyclase results in increased cyclic guanylyl monophophate, cerebral vasodilation, and loss of consciousness
What state must the iron molecule in Hb be in to bind oxygen?
Must be maintained in the ferrous (Fe2+) state for Hb to bind oxygen
Methemoglobin (metHb)
An inactive form of Hb created when the iron molecule of Hb is oxidized to the ferric (Fe3) state because of oxidative damage within the red blood cell
Gives the red blood cell a darker brown color and results in dusky cyanotic or chocolate-colored mucous membranes
Increases the affinity for oxygen in the remaining ferrous moieties of the Hb molecule, decreasing the release of oxygen to the tissues and shifting the oxyhemoglobin dissociation curve to the left
Sulfhemoglobin
Most uncommon dyshemoglobin
May be caused by exposure to high levels of sulfur from drugs (sulphonamides such as sulfasalazines) or other compounds
Stable green pigment
Formed when iron is oxidized from the ferrous (2+) to the ferric (3+) form by drugs or chemicals that contain sulfur
Sulfur atom irreversibly binds to the porphyrin ring of Hb
Incapable for carrying oxygen
Prevents oxygen transport
Shifts the oxyhemoglobin dissociation curve to the right
Can lead to cyanosis without clinical signs of respiratory distress
No antidote
Is irreversible so remains attached to the Hb for the life span of the RBC
Oxidation in the Erythrocyte
Reactive species derived from oxygen can cause oxidative damage within the body by transferring or extracting an unpaired electron to or from another molecule
Protective mechanisms that prevent or reverse oxidative damage include proteins that act as free radical scavengers and reducing agents that can remove the unpaired electron from an oxidized molecule
Erythrocytes are especially vulnerable to oxidative damage because they carry oxygen, are exposed to various chemicals in plasma, and have no nucleus or mitochondria
Hb can undergo autooxidation as an electron is pulled off the Hb and onto an oxygen molecule, resulting in generation of metHb and O2-
Free radicals also may extract electrons by oxidizing deoxyhemoglobin
Oxidant toxins can donate an electron to oxyhemoglobin, creating metHb and H2O2
What are oxidants that are continuously generated in vivo?
Hydrogen peroxide (H2O2)
Superoxide free radicals (O2-)
Hydroxyl radicals (OH-)
What are mechanisms that erythrocytes have to protect themselves from oxidative damage?
Superoxide dismutase
Catalase
Glutathione peroxidase
Glutathione
metHb reductase
Reducing agents such as NADPH and NADH are instrumental in reducing oxidized glutathione and metHb back to functional molecules
Heinz Bodies
Aggregates of denatured, precipitated Hb molecules within erythrocytes that form as Hb with oxidative damage is metabolized
Oxidation of the SH groups of Hb causes conformational changes in the globin chain that result in precipitation of the denatured globin
Aggregates of denatured globin and metabolized metHb clump into Heinz bodies and continue to coalesce until visible, pale structures can be seen within the red blood cell cytoplasm
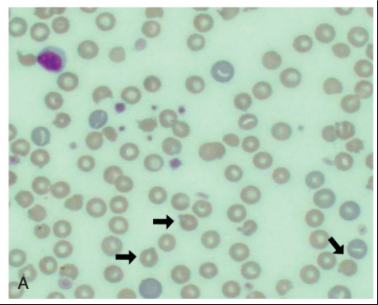
What do Heinz bodies have an affinity for?
Membrane proteins
Binding of Heinz bodies to these proteins causes disruption of anion transport, decreased membrane deformability, and aggregations of membrane protein complexes that may act as autoantibodies
Ghost Cells
Numerous Heinz bodies can disrupt the membrane sufficiently to result in ghost cells - empty red blood cells with just a membrane and Heinz body remaining
Associated with oxidation-induced intravascular hemolysis
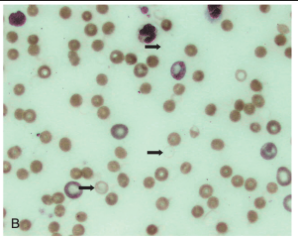
How are erythrocytes that have undergone oxidative damage dealt with by the body?
They are removed by the mononuclear phagocyte system, particularly within the spleen
What are causes of methemoglobinemia in small animals?
Acetaminophen ingestion
Topical benzocaine products
Phenazopyridine ingestion
Nitrites
Nitrates
Skunk musk
Hydroxycarbamide
Aniline
metHb reductase deficiency
What are things that cause methemoglobinemia in humans?
Dapsone
Metoclopramide
Sulfonamides
Nitroglycerine
Nitroprusside
What are substance that cause hemolytic anemia secondary to Heinz bodies but also methemoglobinemia?
Allium plants
Propylene glycol
Zinc
Methylene blue
Crude oils
Naphthalene
Repeated use of propofol in cats
Phenothiazine
Phenylhydrazine
Methionine in cats
Menadione (vitamin K3) in dogs
Copper
What are the pathways by which acetaminophen is metabolized in the liver?
Conjugated to a sulfate compound by a phenol sulfotransferase
Conjugated to a glucuronide compound by a uridine diphosphate-glucuronosyltransferase
Can be transformed and oxidized by the cytochrome P-450 system that converts it to the reactive intermediate, NAPQI
The glucuronide and sulfate conjugations are nontoxic and excreted in the bile and urine in most species other than the cat
GSH reacts with NAPQI to form a nonreactive molecule, mercapturic acid, which is excreted in the urine
An additional metabolic of acetaminophen, para-aminophenol (PAP), is produced by deacylation of acetaminophen by hepatic microsomal carboxyesterases
PAP is removed by biotranformation through N-acetylation with N-acetyltransferase (NAT), conjugation with GSH, or sulfation
How do higher doses of acetaminophen cause toxicity?
Low doses are metabolized to nontoxic products, but higher doses can overwhelm the sulfate and glucuronide conjugate systems of the liver and deplete GSH stores
NAPQI and PAP build up and unmetabolized acetaminophen accumulates
Half-life of acetaminophen becomes longer with higher doses
NAPQI oxidizes hepatic proteins, resulting in hepatocellular damage
PAP may play a more significant role in erythrocyte oxidative damage
PAP cooxidizes with oxyhemoglobin forming metHb and an oxidized PAP intermediate
The intermediate is reduced by GSH and the metHb is reduced primarily by metHb reductase
Methemoglobinemia becomes overt when metHb reductase and necessary reducing equivalents become depleted in erythrocytes
After the acute episode of metHb production, Heinz bodies being to form and aggregate into larger structures, eventually causing enough changes in the erythrocyte to trigger hemolysis
Prognosis for Acetaminophen Toxicity
Prognosis for acetaminophen toxicity is guarded
Time from ingestion to treatment is the most important factor in determining morbidity and survival
Methemoglobinemia Secondary to Topical Benzocaine
Benzocaine sprays for laryngeal spasm in cats and over-the counter creams for pruritis in dogs and cats have been associated with methemoglobinemia
Metabolites of benzocaine are likely responsible for oxidative damage to Hb
Methemoglobinemia Secondary to Skunk Musk
One report of methemoglobinemia and Heinz body hemolytic anemia in a dog after exposure to skunk musk
Toxic substance in skunk musk thought to be thiols, which can react with oxyhemoglobin to form met Hb, a thiyl radical, and H2O2
Methemoglobinemia Secondary to Nitrites and Nitrates
Not documented to cause Heinz body production
Can occur after receiving vasodilatory drugs that release nitric oxide, including nitroglycerine and sodium nitroprusside
Nitric oxide is decomposed by interacting with oxyhemoglobin to form metHb and nitrate
metHb is reduced by metHb reductase in RBCs, but evidence indicates that NO decreases metHb reductase activity
NO also interacts with oxygen to form nitrogen dioxide, which dissolves in solution to yield nitrite and nitrate
Nitrite can convert oxyhemoglobin to metHb
Toxicity should be considered in animals receiving these drugs and developing an unexplained hyperlactatemia
Methemoglobin Reductase Deficiency
Rare congenital abnormality
Affected animals cannot efficiently reduce metHb so have elevated blood levels of metHb, exhibit mild to moderate cyanosis of the mucous membranes, and may suffer from exercise intolerance
Definitive diagnosis by measuring erythrocyte metHb reductase enzyme activity at a research laboratory
Condition is fairly benign and rarely requires treatment
Indications for treatment include clinical signs of metHb such as lethargy, tachycardia, and/or tachypnea, and as preparation for animals that require general anesthesia
Clinical Signs of Carbon Monoxide Toxicity
Lethargy
Depression
Headache
Confusion
Syncope
Seizures
Unconsciousness
Death
Tachypnea
Tachycardia
Nausea
Dysrhythmias
Vomiting
Cherry red mucous membranes
Severity does not correlate consistently with COHb levels
Clinical Signs Associated with COHb Levels >15% in Humans
Overt signs of toxicity such as tachypnea and headache
Clinical Signs Associated with COHb Levels >30% in Humans
Neurologic dysfunction
Clinical Signs Associated with COHb Levels >50% in Humans
Loss of consciousness that can progress to apnea and death
Delayed Neuropsychiatric Syndrome (DNS)
Delayed neuropsychiatric syndrome (DNS) described in humans and some veterinary cases of CO toxicity
Develops 3-240 days following the toxic episode
Clinical signs in veterinary patients range from ataxia to inability to ambulate, depressed or stuporous mentation, seizures, and deafness
Risk factors in humans include age (older), duration of unresponsiveness, and history of illness
At what level of metHb do clinical signs begin?
20%
Clinical Signs of Methemoglobinemia/Sulfhemoglobinemia
Tachycardia
Tachypnea
Dyspnea
Lethargy
Anorexia
Vomiting
Weakness
Ataxia
Stupor
Hypothermia
Ptyalism
Convulsions in cats
Coma and death with metHb levels reach 80%
Chocolate brown MM and cyanosis
Cyanosis appears at metHb levels of 12-14% or more
Clinical signs of sulfhemoglobinemia are very similar to metHb
What do definitive diagnosis and quantification of COHb, metHb, and sulfhemoglobinemia levels require?
Direct measurement via a co-oximeter or assay
Co-Oximeter
Machine used to measure Hb content, oxygen saturation, percentage of COHb, and percentage of metHb by differentiating wavelength absorbance values
Assay for metHb and Sulfhemoglobinemia
Assay for metHb and sulfhemoglobinemia involves spectrophotometrically quantifying the change in absorbance at 630 nm before and after the addition of cyanide to the sample
Cyanide converts metHb to cyanmethemoglobin, which has a different absorbance than metHb
Sulfhemoglobin has a similar spectral peak as metHb, making it appear as elevated met Hb levels on many co-oximeter readings
Sulfhemoglobin can be distinguished from metHb by adding cyanide as the spectral peak will persist with sulfhemoglobin, indicating the presence of a different dyshemoglobin
H2O2 can be added as well as it will bind to sulfHb
Comparing Pulse Oximeter Oxygen Saturation to Arterial Blood Gas Saturation (Saturation Gap) for Diagnosing Carboxyhemoglobinemia, Methemoglobinemia, and Sulfhemoglobinemia
Pulse oximeter determines the ratio of oxyhemoglobin to deoxyhemoglobin
Presence of metHb or sulfhemoglobinemia distorts the ratio
If metHb levels exceed 30% or sulfhemoglobinemia levels exceed 28%, the pulse oximeter reading plateaus around 85% regardless of the true oxygen content
A dyshemoglobinemia should be suspected if the saturation gap is greater than 5%
Evaluating a Peripheral Blood Smear for Oxidative Damage
Examining a peripheral blood smear for Heinz bodies, eccentrocytes, and ghost cells can be helpful when looking for evidence of oxidative damage
May require a stain such as new methylene blue or a reticulocyte stain
Treatment for Carbon Monoxide Toxicity
Elimination of CO depends on minute ventilation, duration of exposure, and the fraction of inspired oxygen
Oxygen therapy is the mainstay of treatment
Increasing the amount of oxygen in the blood decreases the half-life of CO as dissolved O2 competes with CO for Hb binding sites
CO is then displaced from Hb and exhaled through the lungs
Oxygen rates at 50-150 ml/kg/min
Also indicated to prevent DNS because mechanism is thought to be related to hypoxia and reperfusion
Limiting the degree and duration of hypoxia has become the goal of treatment to prevent DNS
Hyperbaric oxygen therapy debated
Other than oxygen therapy, the bulk of treatment is supportive care
Active warming
Ensuring adequate tissue perfusion
Since oxidative damage has a role in development of DNS, antioxidantes such as N-acetylcysteine and vitamin E may be useful in moderate to severe CO toxicity and/or those that develop signs of DNS
Prognosis for Carbon Monoxide Toxicity
Initial unconsciousness or severe neurologic abnormalities are associated with a more guarded prognosis
Overall prognosis is fair with time and supportive care
Treatment for Oxidative Damage to Erythrocytes
Oxygen therapy increases the amount of dissolved oxygen in the blood, but once the Hb capable of carrying oxygen are maximally saturated, supplemental oxygen is not sufficient as a sole therapy
Treatment for methemoglobinemia often involves diuresis or medications that increase the rate of elimination or decrease the production of toxic metabolites
Induction of vomiting followed by activated charcoal is indicated if the animal has a history of recently ingesting a toxic substance and is not yet clinically ill
Supportive care
Therapy for severe sulfhemoglobinemia may also include packed red blood cell transfusions
Treatment for Acetaminophen Toxicity
N-Acetylcysteine (NAC)
Preferred treatment for acetaminophen toxicity
Augments the endogenous glutathione stores as it is hydrolyezd to cysteine
NAC also interacts directly with NAPQI to form a nontoxic conjugate and increases the fraction of acetaminophen excreted as the sulfate conjugate
Most effective if administered within 12 hours of ingestion but still recommended up to 36-80 hours after ingestion
Recommended regimen is an initial dose of 140 mg/kg IV (280 mg/kg in severe toxicosis) followed by 70 mg/kg q6h for 7 additional treatments
Recommend a slow IV infusion of a 5% solution over 30-60 minutes
Typically causes nausea and vomiting when given orally, hypotension and bronchospasm if given rapidly intravenously, and phlebitis if it leaks perivascularly
Methylene Blue for Treatment of Oxidative Damage to Erythrocytes
Increases the rate of reduction of metHb through use of another reducing system within the erythrocyte, NADPH dehydrogenase
Administered as a 1% solution intravenously over several minutes at 1 mg/kg once
Improvement in clinical parameters should be noted within 30 minutes of administration
Methylene blue causes oxidative damage in RBCs and can potentiate a Heinz body anemia caused by the original oxidative insult
A delayed reaction may occur so hematocrit and blood smear should be monitored for 3-4 days after administration
Clinical signs attributable to sulfhemoglobinemia will not improve after methylene blue
Adjunctive Treatments for Dyshemoglobinemias
Ascorbic acid
30 mg/kg IV q6h as an antioxidant
Can augment metHb conversion to HB through nonenzymatic reduction
Cimetidine
Histamine-2 receptor antagonist
Useful in cases of acetaminophen toxicity because it inhibits the P-450 oxidation system in the liver, limiting the production of NAPQI
Studies haven't demonstrated consistent benefits
5 mg/kg IV q8h
SAMe
Essential metabolite that is vital to hepatocytes - hepatoprotective, antioxidant properties, and decreases the osmotic fragility of erythrocytes
Bioflavonoids
Antioxidants that work by increasing the activity of the NADPH reductase system
Blood transfusion
May be necessary in patients with severe hemolytic anemia secondary to Heinz body production and for sulfhemoglobinemia