Rutherford's Gold Foil Experiment
1/10
Earn XP
Description and Tags
Study on alpha particle scattering
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What was the Rutherford Gold Foil Experiment?
An experiment that consisted of beams of high-energy alpha particles fired at thin gold foil and a detector on the other side to determine:
the different angles of deflection of the alpha particles
The number of alpha particles that were deflected at each angle
What was the apparatus?
A source of alpha particles in a lead container
A thin sheet of gold foil
A movable detector
An evacuated chamber
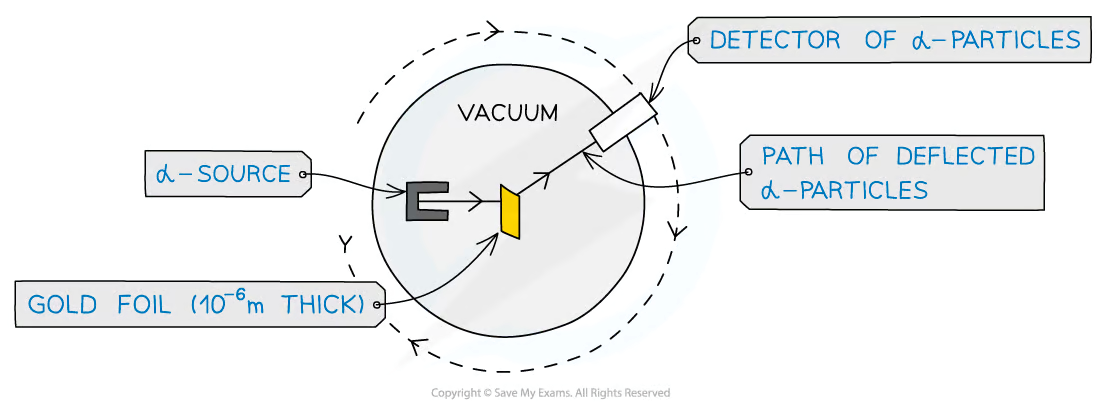
What was the purpose of the lead container?
To produce a collimated beam of alpha particles as alpha particles are absorbed by the lead, therefore will travel through the long narrow hole at the front allowing a concentrated beam
What is meant by collimated beam?
A beam of light (or particles) in which the rays are made to travel parallel to each other
What was the purpose of the thin sheet of gold foil?
Had to be thin enough in order to ensure the aloha particles would not stop completely. Gold was chosen due to its malleability, therefore was easy to hammer into thin sheets
What was the purpose of the evacuated chamber?
Ensured the alpha particles did not collide with any particles on their way to the foil target
What were the findings from the experiment?
An alpha particle is the nucleus of a helium atom, so it has a positive charge
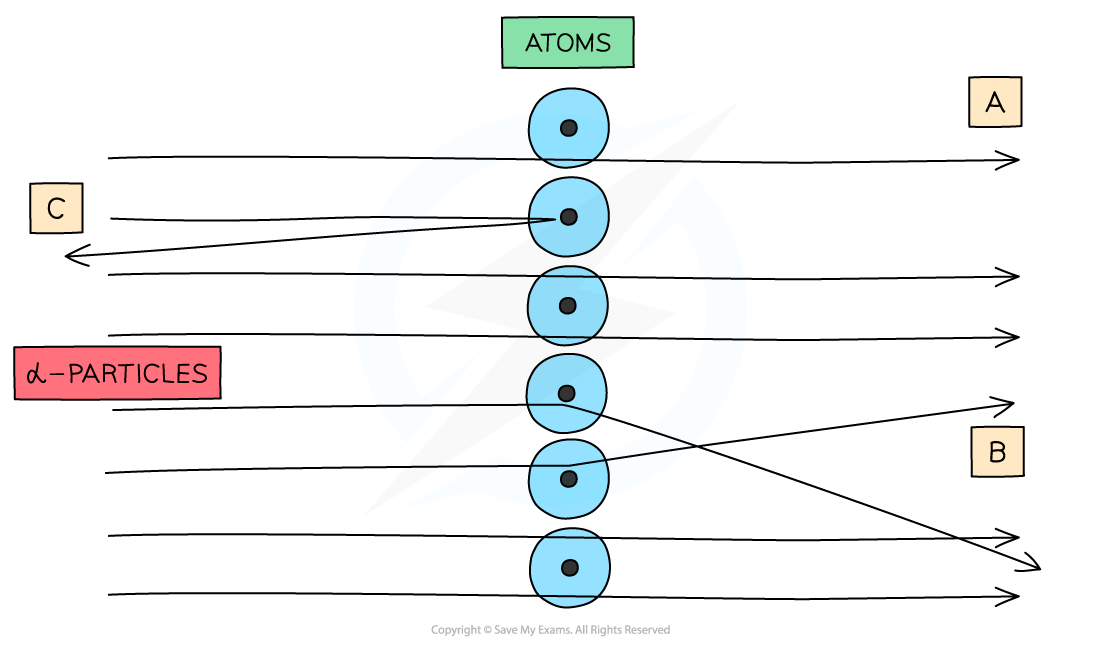
What were the observations made at point A?
The majority of alpha particles passed straight through the foil undeflected
Suggests the atom is mostly empty space
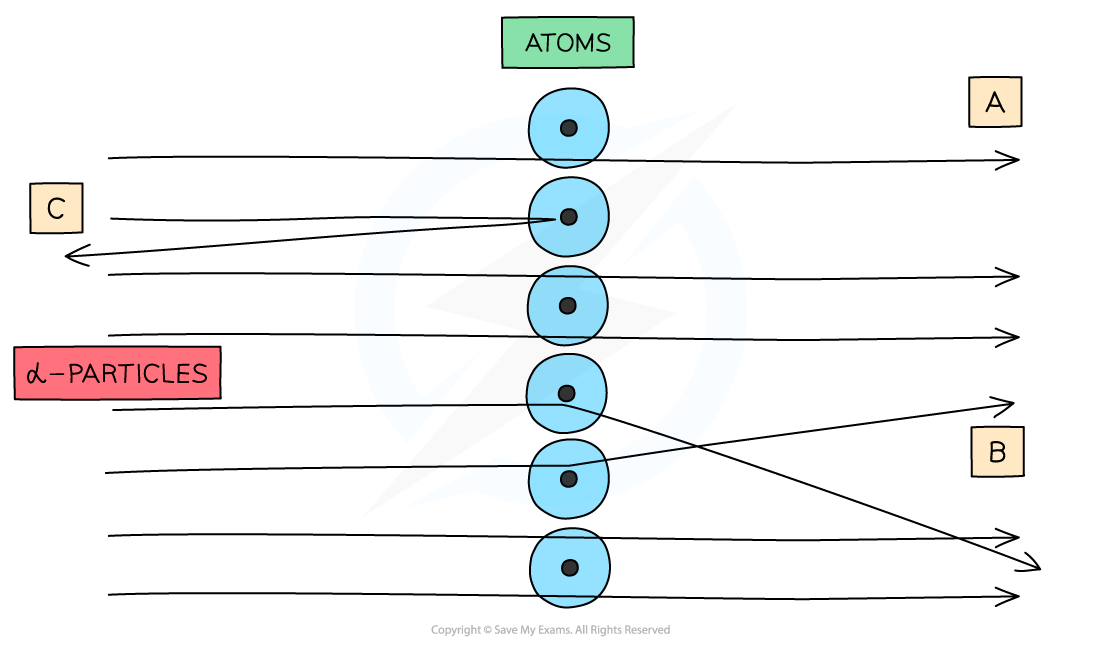
What were the observations made at point B?
Some alpha particles deflected through small angles less than 10 degrees
Suggests there is a positive nucleus at the center (since two positives would repel)
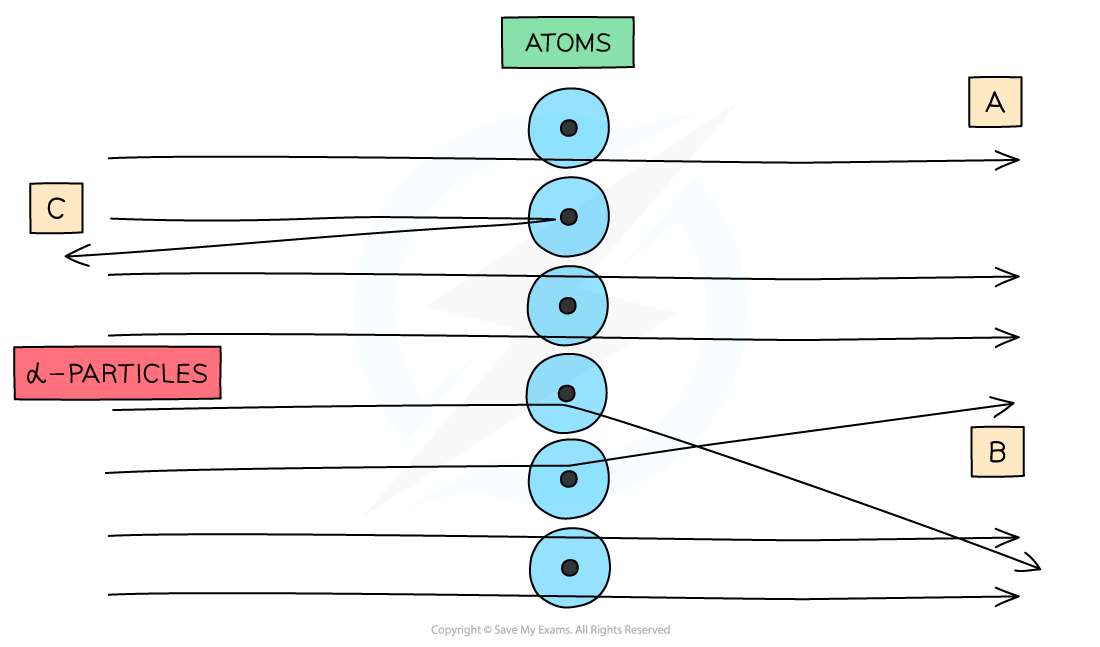
What were the observations made at point C?
Only a small number of alpha particles deflected straight back at angles of greater than 90 degrees
Suggests the nucleus is extremely small and is where most of the mass and charge of the atom are concentrated
Led to the conclusion that atoms consist of small, dense positively charged nuclei surrounded by negatively charged electrons
What were the overall observations?
Most of the α-particles went straight through the foil
The largest value of n will therefore be at small angles
Some of the α-particles were deflected through small angles
n drops quickly with increasing angle of deflection θ