CHEM12 UNIT 1
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Photon
Unit of light energy
Quanta
Small amount of energy
Electron
A negatively charged subatomic particle
Radioactivity
Spontaneous decay or disintegration of the nucleus of an atom
Nucleus
the dense center of an atom with a positive charge
proton
a positively charged subatomic particle
neutron
an electrically neutral subatomic particle
isotopes
atoms with the same number of protons but different number of neutrons
atomic number (Z)
number of protons in a nucleus
mass number (A)
protons + neutrons in a radioisotope (an isotope that emits radioactive gamma rays and/or subatomic particles
photoelectric effect
electrons are emitted by matter that absorbs energy from shortwave electromagnetic radiation (visible/UV light)
ground state
lowest energy state of an atom
quantized
energy moves in distinct steps (levels) rather than moving continuously E
excited state
state where potential energy is higher than ground state
Dalton’s model of an atom
“Billiard ball”
Matter is made of small, invisible spheres
Matter cannot be created, destroyed or divided
Atoms of the same element are identical in all other properties
Thomson’s model of an atom
Atoms are positively charged spheres with negatively charged particles embedded in them
Rutherford’s model of the atom
Positively charged, dense, nucleus with electrons around it
Shining alpha particles at golden foil, most went through but some had wild deflections
Successes of the Bohr model
Lower energy levels filled first
First energy level has max 2 electrons, second has max 8, third has max 18
Atoms are arranged according to photons, neutrons, and electrons and elemental properties
Electrons exist in discrete energy levels
Failures of the Bohr Model
Confusing past the first 20 elements
Energies are inconsistent for atoms with more than 1 electron
Electrons don’t orbit the nucleus
Bohr’s model of the atom
circular orbits with distinct energy levels
only exists in allowed orbits
can jump orbits by gaining or losing energy
Einstein’s contributions to the atom
Electromagnetic radiation is a stream of photons, each photons has it's own quantum energy
Energy changes at the atomic level only occur at a certain amount of energy
Energy of a photon is transferred to the electron, breaking the electron free from the atom
A minimum amount is required to be met
Photon strength depends on energy frequency
Limitations of Rutherford’s model
if an atom is constantly accelerating, it should be emitting electromagnetic energy
If it is constantly emitting photons, it should be losing energy and decaying into a nucleus
Spectroscopy
Analyzing spectrums to determine properties of their source
line/emission spectrum
unique sets of colour produced when light from an excited substance is passed through a prism
Quantized
It can only exist in discrete, specifics values rather than a continuous range
Quantum mechanics
Application of quantum theory to explain properties of matter
Orbital
Region around nucleus where electron has a high probability of being found
Electron probability density/electron probability distribution
Indicates regions with the greatest probability of finding an electron ,determined with wave equations
Quantum mechanica model
Model for the atom based on quantum theory and calculation of probabilities for the location of electrons.
Heisenberg’s uncertainty principle
The idea that it is impossible to determine the position and speed of an object at the same time due to it’s nature of being both a. wave and a particle.
Pauli exclusion principle
Electrons placed in lowest orbitals first, arrows point in opposite directions to indicate spin.
No two electrons in the same atom can be in the same quantum state, therefore no two electrons have the same four quantum numbers and orientation is a unique property.
Aufbau principle
Orbitals must be filled before moving to the next highest orbital
Hund’s rule
Place one electron in each orbital (letter) of the same before a second electron is added
Order of orbital placement
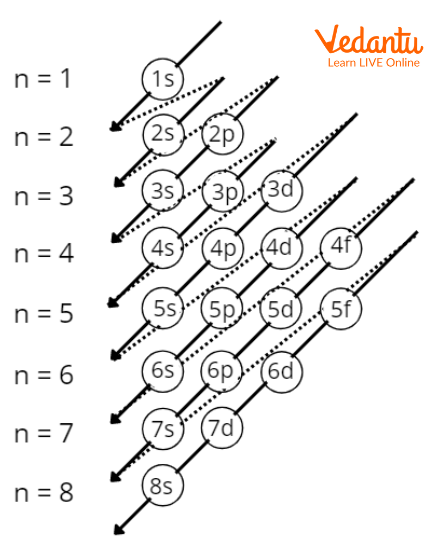
Analogous electron configurations
Typically found in d subshell
Half filled and filled subshells are more stable than unfilled shells
Atoms that end in d^4 or d^9 will promote an electron from an s orbital
Cr, Mo, W, Cu, Ag, Au
Paramagnetism
weak attraction/magnetic field due to unparied electrons in an atom
Unpaired electrons all have the same spin (Hund’s) spinning generates magnetic field
Quantum numbers
Describes the quantum mechanical properties of orbitals
Principal quantum number (n)
Size and energy level (shell) of an orbital
eg: (n)s
Ranges from n=1 to n=∞
Secondary quantum number (l)
Describes shape of orbital
Ranges from 0 to (n-1)
l=0 orbital s
l=1 orbital p
l=2 orbital d
l=3 orbital f
magnetic quantum number (ml)
Orientation of orbital
(2l+1) possibilities ranging from -l to l
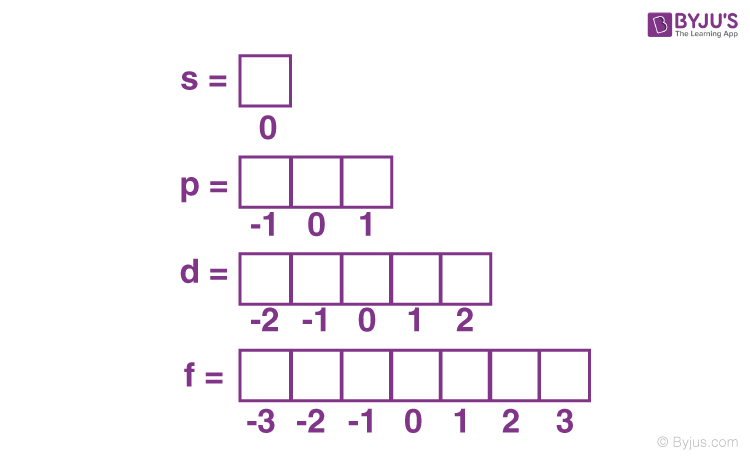
spin quantum number (ms)
Orientation of the axis of electron spin
Can only be +½ or -½
Draw out diagram, if spin is facing downwards it has a negative axis