Organic Compounds with Oxygen, Halogen, Sulfur: Nomenclature, Classification, and Reactions
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
159 Terms
Alcohol
A functional group is a specific structural arrangement of atoms or bonds that imparts a characteristic chemical reactivity to the molecule.
Alcohol functional group
--OH
IUPAC naming for alcohols
In the IUPAC system, name by replacing the e with ol.
Naming Organic Compounds
Name the parent as the longest continuous chain of carbon atoms THAT CONTAINS THE FUNCTIONAL GROUP.
Substituent numbering
Name and number substituents GIVING THE CARBON OF THE FUNCTIONAL GROUP THE LOWEST NUMBERS.
Alphabetical order of substituents
Place the names of the substituent groups in alphabetical order before the name of the parent compound.
Identical groups indication
The number of identical groups is indicated by the Greek prefixes di-, tri-, tetra-, and so on.
Ethylene glycol
Ethylene glycol is considered a double alcohol because it has one OH group at each end.
Uses of ethylene glycol
It is an organic molecule that is a useful industrial compound found in many consumer products, including automotive antifreeze, ballpoint pens, paints, plastics, and cosmetics.
Taste and odor of ethylene glycol
Ethylene glycol has a sweet taste and is often accidentally or intentionally ingested. It is odorless.
Toxic effects of ethylene glycol
It and its toxic byproducts first affect the central nervous system (CNS), then the heart, and finally the kidneys.
Fatal ingestion of ethylene glycol
Ingestion of sufficient amounts can be fatal.
Effects on Dr. George Blumenschein
Within four hours of being poisoned, he began experiencing slurred speech, poor balance and a loss of fine motor skills.
Health complications from poisoning
He was later found to have central nervous system depression, cardiopulmonary complications and renal failure.
Kidney function post-poisoning
His lifetime was shortened by the poison as he now has only 40% kidney function.
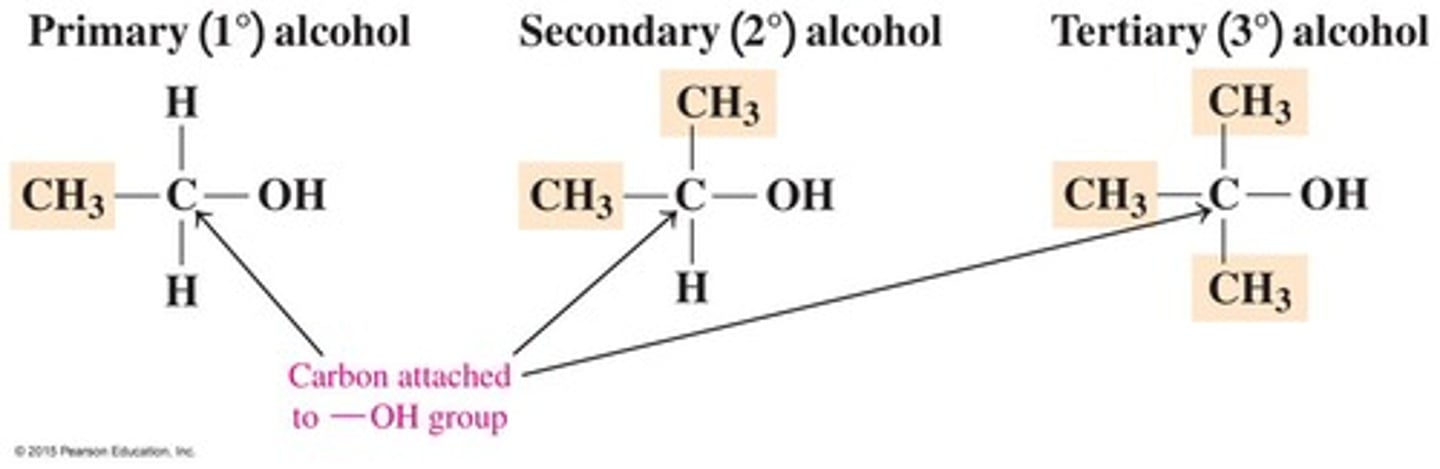
Classification of Alcohols
Alcohols are classified by the number of alkyl groups attached to the carbon bonded to the hydroxyl as primary (1°), secondary (2°), or tertiary (3°).
Primary alcohol
An alcohol where the hydroxyl group is attached to a carbon that is bonded to only one other carbon.
Secondary alcohol
An alcohol where the hydroxyl group is attached to a carbon that is bonded to two other carbons.
Tertiary alcohol
An alcohol where the hydroxyl group is attached to a carbon that is bonded to three other carbons.
Thiol functional group
--SH
IUPAC naming for thiols
In the IUPAC system, name by replacing the e with thiol.
Thiols
Thiols readily bond with proteins.
Proteins
Proteins are found in human skin, therefore the thiols and their smell binds with the skin proteins.
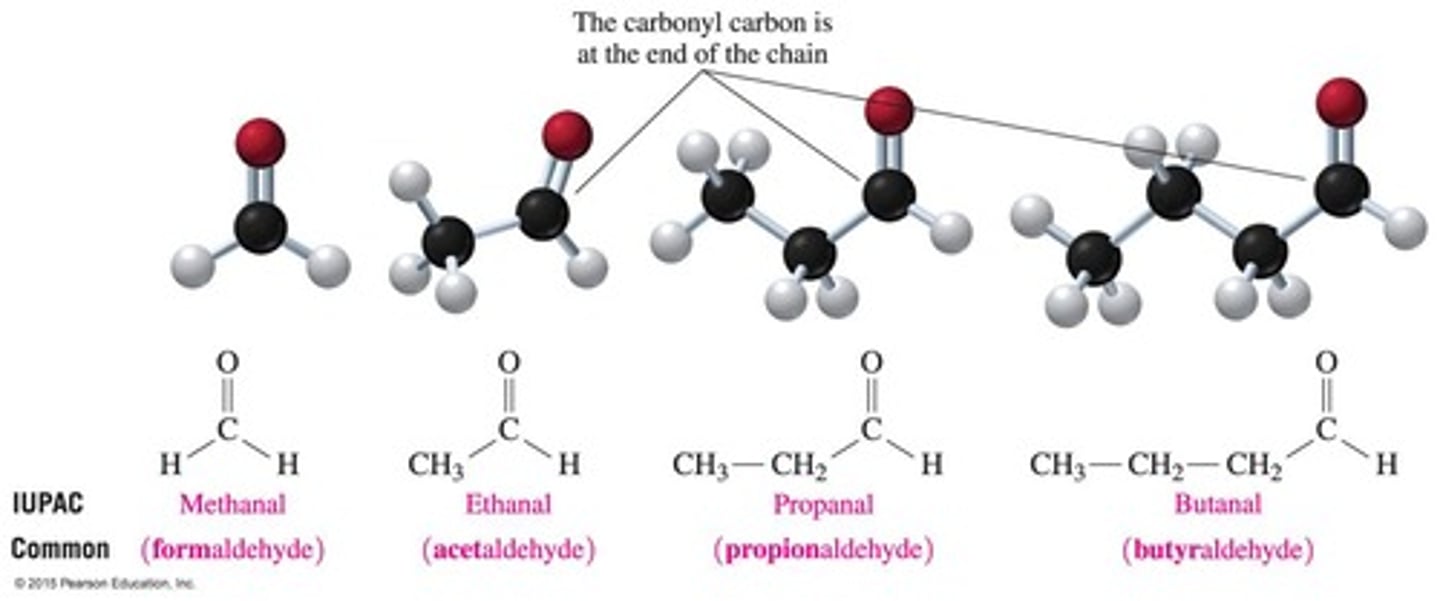
Aldehyde
In an aldehyde, the carbonyl group is at the end. In the IUPAC system, name by replacing the e with al.
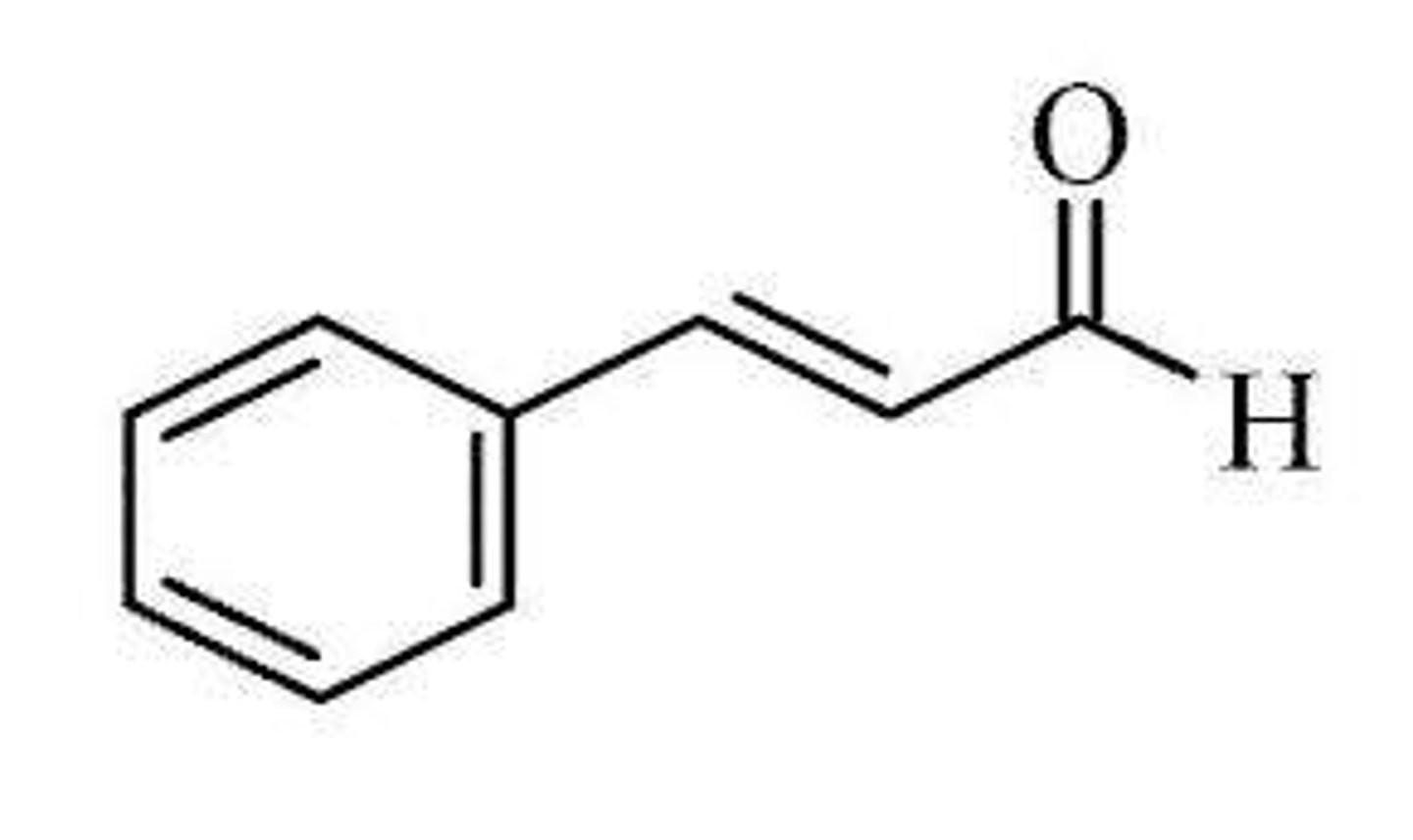
Cinnamaldehyde
Cinnamon has a molecular formula of C₉H₈O which gives cinnamon bark the name 'Cinnamaldehyde'.
Cellulose
Cellulose is an insoluble substance for human beings and is the main constituent of plant fibers, such as cotton.
Polysaccharide
Cellulose is a polysaccharide consisting of chains of glucose monomers.
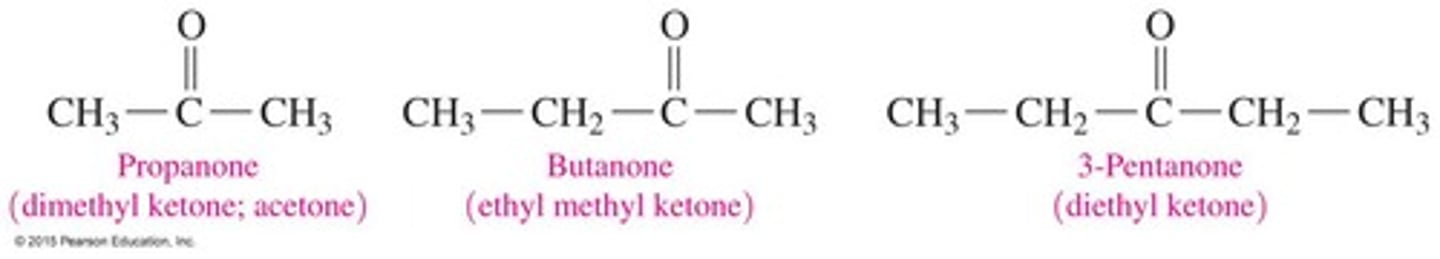
Ketone
In a ketone, the carbonyl group is in the middle of the chain. In the IUPAC system, name by replacing the e with one.
Solubility in Water
A substance must be polar to be soluble in water.
Alcohols
Alcohols contain polar —OH groups and form hydrogen bonds with other alcohol molecules and with water.
Solubility of Alcohols
Alcohols that have one to three carbons are soluble in water; the solubility of alcohols in water decreases with increasing number of carbons.
Hydration
Hydration is the process of adding water, specifically adding -H to one carbon and -OH to the second carbon.
Dehydration of an Alcohol
The loss of —H and —OH from adjacent carbon atoms, producing an alkene and water.
Oxidation
Oxidation reactions increase the number of carbon-oxygen bonds by the addition of oxygen or a loss of hydrogen atoms.
Reduction
Reduction reactions reduce the number of bonds between carbon and oxygen atoms.
Oxidation of 1° Alcohols
Primary alcohols are oxidized to produce an aldehyde.
Solubility of Aldehydes and Ketones
Aldehydes and ketones form hydrogen bonds with water molecules and are very soluble when they have four or fewer carbons.
Hydration Reaction
Adding water to an organic compound.
Dehydration Reaction
The process that produces an alkene and water from an alcohol.
Intermolecular Forces
The forces that exist between molecules, affecting their physical properties.
Dipole-Dipole
A type of intermolecular force that occurs between polar molecules.
Hydrogen Bonding
A strong type of dipole-dipole interaction that occurs between molecules containing hydrogen bonded to highly electronegative atoms.
Oxidation of 2° Alcohols
Secondary alcohols are oxidized to produce a ketone.
Oxidation of 3° Alcohols
Tertiary (3°) alcohols do not readily oxidize because there is no hydrogen atom on the carbon bonded to the —OH group.
Reduction of Aldehydes and Ketones
The number of carbon-oxygen bonds is reduced by the addition of hydrogen or the loss of oxygen. Aldehydes reduce to form primary alcohols, and ketones reduce to form secondary alcohols.
Oxidation of Thiols
When thiols undergo oxidation, an H atom is lost from each of two —SH groups; the product is a disulfide.
Disulfide Bonds
Protein in hair is cross-linked by disulfide bonds found in the amino acid cysteine.
Combustion Reaction
In the combustion reaction 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g), C2H6 is oxidized and O2 is reduced.
Isomers
Structural isomers have the same number and type of atoms but different arrangements.
Stereoisomers
Stereoisomers include cis/trans and optical isomers (chiral molecules, enantiomers).
Chiral Molecules
Chiral molecules are non-superimposable and occur when 4 different groups are bonded to a carbon atom, yielding mirror images.
Thalidomide-Induced Phocomelia
A congenital skeletal disorder that affects the limbs, caused by thalidomide taken by a mother during pregnancy.
IUPAC Naming
Naming alcohols, thiols, ethers, aldehydes, and ketones involves writing the IUPAC names and drawing the condensed structural formulas.
Dehydration of Alcohols
Involves writing balanced chemical equations for the dehydration of alcohols.
Oxidation of 2-Propanol
The primary product for the reaction of 2-propanol when it undergoes oxidation is acetone.
Reduction of 3-Ethyl Heptanal
The product of reduction of 3-ethyl heptanal is 3-ethyl-1-heptanol.
Combustion of Propanol
The products when propanol undergoes a combustion reaction are CO2, H2O, and energy.
Oxidation Product of 3-Pentanol
The IUPAC name of the oxidation product of 3-pentanol is 2-pentanone.
Combustion Reaction of Alcohol
A flaming dessert is prepared using heat from the combustion of an alcohol: 2CH3—CH2—OH(g) + 6O2(g) → 4CO2(g) + 6H2O(g) + energy.
Oxidation of Butanal
Oxidation of two molecules of butanal results in the formation of a carboxylic acid.
Reduction of Butanal
Reduction of two molecules of butanal results in the formation of primary alcohols.
Ethers
Compounds that have two alkyl groups bonded to an O atom.
Alkyl halides
Compounds that contain a halogen atom X (X = F, Cl, Br, or I).
Carbonyl group
A functional group characterized by a carbon atom double-bonded to an oxygen atom.
Primary (1°) alcohol
An alcohol with an OH group on a carbon bonded to one carbon.
Secondary (2°) alcohol
An alcohol with an OH group on a carbon bonded to two carbons.
Tertiary (3°) alcohol
An alcohol with an OH group on a carbon bonded to three carbons.
Physical Properties of Alcohols
Alcohols have an H atom bonded to an O atom, allowing for intermolecular hydrogen bonding.
Boiling and melting points of alcohols
Alcohols have higher boiling and melting points than hydrocarbons of comparable size and shape.
IUPAC nomenclature of alcohols
Alcohols are identified by the suffix -ol.
Naming an alcohol
Find the longest carbon chain containing the C bonded to the OH group and change the -e ending of the parent alkane to the suffix -ol.
Numbering the carbon chain in alcohols
Number the carbon chain to give the OH group the lower number.
OH group in cyclic compounds
When an OH group is bonded to a ring, the OH is automatically on C1, and the "1" is usually omitted from the name.
Ether
An ether has an O atom with a bent shape.
Intermolecular Hydrogen Bonds
Ethers do not contain an H atom bonded to an O atom, so two ethers cannot form intermolecular hydrogen bonds.
Intermolecular Forces of Ethers
Ethers have stronger intermolecular forces than alkanes and weaker intermolecular forces than alcohols.
Melting and Boiling Points of Ethers
Ethers of comparable size and shape tend to have higher melting and boiling points than hydrocarbons and lower melting and boiling points than alcohols.
Ethanol
Ethanol is the alcohol present in alcoholic beverages, and it is formed from the fermentation of carbohydrate chains.
2-Propanol
2-Propanol (isopropyl alcohol) is the major component of rubbing alcohol, which is used to sterilize skin and medical instruments.
Glycerol
Glycerol is a triol used in lotions, liquid soap, and shaving cream; it is sweet tasting, but nontoxic, so it can be used in food products.
Diethyl Ether
Diethyl Ether is a general anesthetic that dramatically changed surgery in the nineteenth century; it is safe and easy to administer, but highly flammable and causes nausea.
Oxidation Reagent
The symbol [O] indicates an oxidation reagent has been added.
Primary Alcohol Oxidation
Primary alcohols first oxidize to aldehydes (RCHO), replacing 1 C—H with 1 C—O.
Aldehyde Oxidation
Aldehydes are further oxidized to carboxylic acids (RCOOH), replacing 1 C—H with 1 C—O.
Secondary Alcohol Oxidation
Secondary alcohols are oxidized to ketones.
Tertiary Alcohol Oxidation
Tertiary alcohols have no H atoms on the C with the OH group, so they are not oxidized.
Ethanol Metabolism
When ethanol is consumed, it is quickly absorbed in the stomach and small intestines; in the liver, the enzymes alcohol and aldehyde dehydrogenase act as oxidizing reagents.
Primary Alkyl Halide
A primary (1°) alkyl halide has a halogen on a carbon bonded to one carbon.
Secondary Alkyl Halide
A secondary (2°) alkyl halide has a halogen on a carbon bonded to two carbons.
Tertiary alkyl halide
A tertiary (3°) alkyl halide has a halogen on a carbon bonded to three carbons.
Intermolecular hydrogen bonding
Alkyl halides are not capable of intermolecular hydrogen bonding.
Boiling and melting points of alkyl halides
They have higher melting and boiling points than similar alkanes, but lower than alcohols.
Factors affecting boiling and melting points
The boiling and melting points of an alkyl halide will increase with the size of the alkyl group and the size of the halogen.
Solubility of alkyl halides
All alkyl halides are insoluble in water.
IUPAC naming of alkyl halides
Step [1]: Find the parent carbon chain containing the halogen.
Example of alkyl halide nomenclature
Answer: 2-chloro-5-methylheptane.
Chloromethane
Chloromethane (CH3Cl) is produced by giant kelp and algae and is found in emissions from volcanoes.
Dichloromethane
Dichloromethane (or methylene chloride) is an important solvent, once used to decaffeinate coffee.
Shape of thiols
Thiols have a bent shape around the S atom.
Boiling and melting points of thiols
Thiols have lower boiling and melting points than similar alcohols.