To determine: i) the concentration of total suspended solids of a sample of water by filtration (in ppm) ii) the concentration of total dissolved solids of a sample of water by evaporation (in ppm) iii) the pH of a sample of water
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
Theory
i) The concentration of total suspended solids (solids that are not dissolved) of a sample of water (in ppm) is measured by filtration
ii) The concentration of total dissolved solids of the previously filtered water in (ppm) is then measured by evaporation
iii) The pH of local tap water is then found
Procedure: i) The concentration of total suspended solids of a sample of water by filtration
➢ The water sample is added to a volumetric flask to an exact known volume
➢ The mass of a piece of clean, dry filter paper is found on an electronic balance
➢ The water sample is filtered through the pre-weighted filter paper into a Buchner funnel and vacuum filtered
➢ The filter paper is dried in an oven to remove any water
➢ The mass of the filter paper is then found again
➢ The initial mass of the filter paper is subtracted from the final mass
➢ The mass of suspended solids in the known volume of water is then converted to concentration of suspended solids in ppm
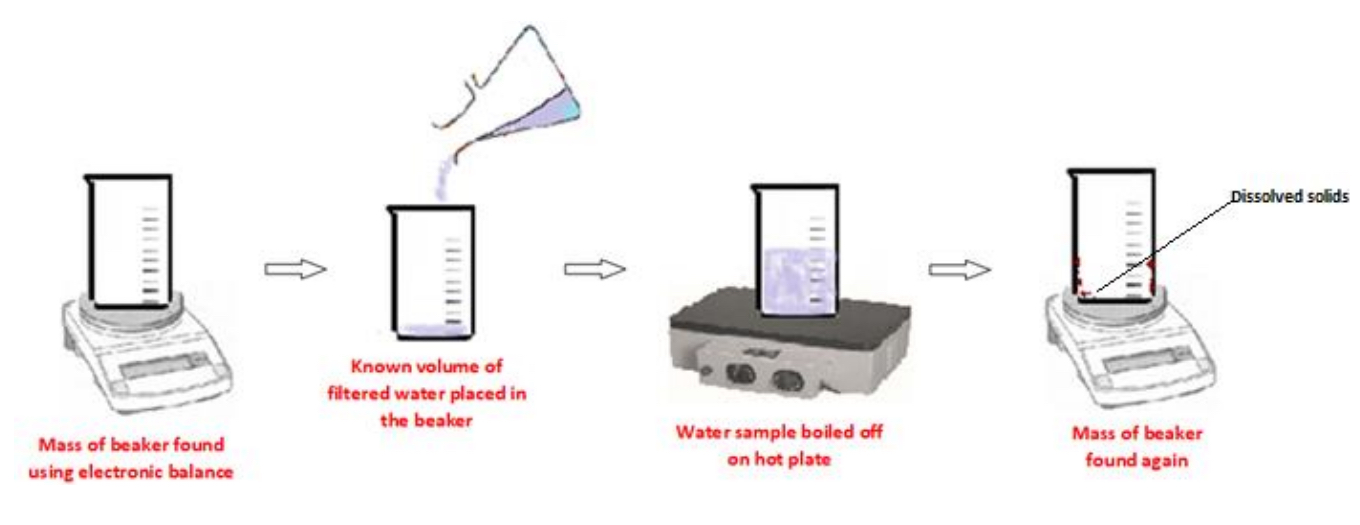
Procedure: ii) The concentration of total dissolved solids of a sample of water by evaporation
➢ The mass of a piece of clean, dry beaker is found on an electronic balance
➢ A known volume of the previously filtered water sample is placed in the pre-weighed beaker
➢ Using a hot plate, the water sample is boiled off/evaporated
➢ When completely dry, the mass of the beaker is then found again
➢ The initial mass of the beaker is subtracted from the final mass
➢ The mass of dissolved solids in the known volume of water is then converted to concentration of dissolved solids in ppm
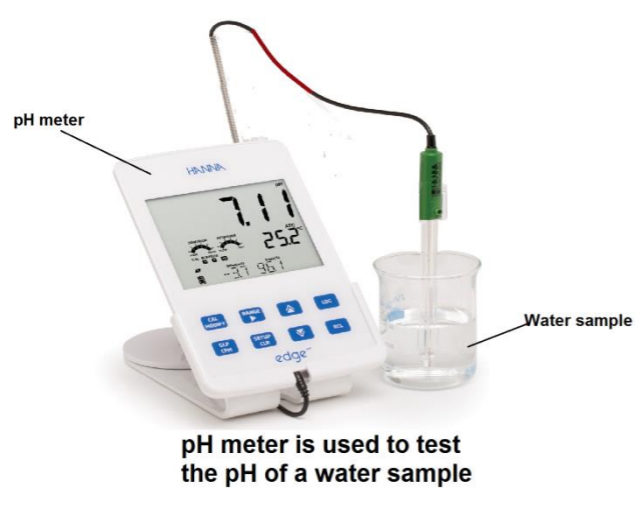
Procedure: iii) the pH of a sample of water
➢ Use a pH meter
➢ Place the probe into the water sample and record the pH
Note: If a pH meter is not available, the pH of the water sample can be measured using universal indicator and the pH scale