15.2/ ES 6- Hydrogen Halides
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Name the two methods that can be used to make hydrochloric acid?
1) Making hydrochloric gas from its elements
2) Making hydrochloric acid as a co-product of PVC production
Explain how hydrochloric acid can be made from its elements?
Done at a plant producing chlorine from brine by electrolysis- chlorine and hydrogen both produced in the process, eliminates hazards with transporting chlorine
Then pass the hydrogen chloride gas through water to dissolve it and produce hydrochloric acid
This can be done on a large scale
What is the advantage of producing hydrochloric acid on the same plant that brine electrolysis occurs on?
Brine electrolysis produces chlorine and hydrogen- both necessary reactants, do not have to transport chlorine- eliminates hazards of transporting chlorine
What is the atom economy when producing hydrochloric acid from hydrogen and chlorine gas?
100%- only one product
What happens to hydrogen chloride when its is dissolved in water?
Dissociates:· Hydrogen chloride gas is made up of covalent molecules- when dissolved in water they form hydrated ions of H+(aq) and Cl-(aq)
Give the chemical equation for the formation of hydrogen chloride from its elements
H2(g) + Cl2(g) --> 2HCl(g)
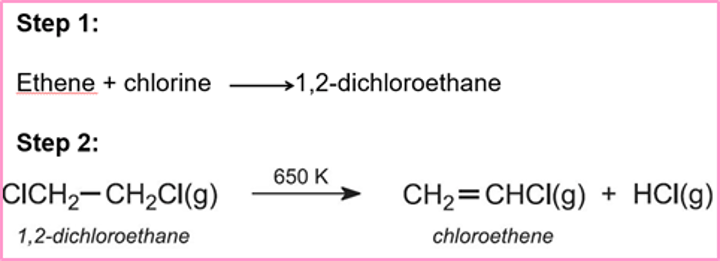
Explain how hydrochloric acid can be produced as a co-product from the chlorination of organic compounds
First stage of the manufacturer of poly(chloroethene) also called poly(vinyl chloride) (PVC) in the reaction of ethene and chlorine
1,2- dichloroethane formed undergoes thermal cracking to give chloroethene and hydrogen chloride
HCl can then be passed through water to make hydrochloric acid
How soluble is HCl in water?
HCl is highly soluble in water and so a high concentration can be easily achieved (due to permanent dipole?)
Draw the repeat unit for PVC or poly(chloroethene)
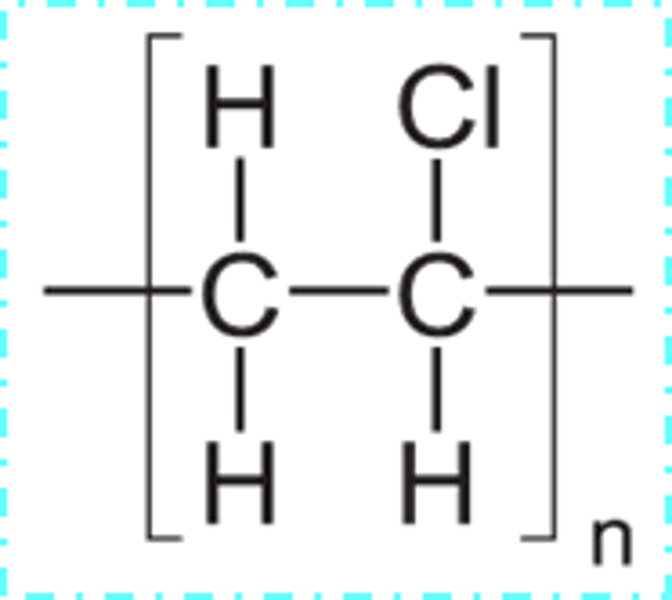
Draw/ give equations for the chemical reactions from ethene to hydrochloric acid in PVC production
What is the atom economy
A measure of the proportion of the reactant atoms that become part of the desired product rather than bi-products in the balanced chemical equation