organic chemistry - aldehydes/ketones
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
suffix of aldehydes
-al
suffix of ketones
-none
What reagent is required to synthesize an aldehyde from a 1 prime alcohol
PCC / CH2Cl2
What reagent is required to synthesize a carboxylic acid from a 1 prime alcohol
CrO3 / dilute acid (H+, H2O)
Oxidizing a 2 prime alcohol with PCC or Jones reagent results in what functional group
alcohol
How would you synthesize a ketone with a benzene ring?
Do Friedel-Craft Acylation (AlCl3 and acid chloride)
Reactions between alcohols and aldehydes/ketones form what type of functional groups?
Describe the mechanism
Hemiacetyls (one alcohol, one ether) and/or acetal (two ethers)
mechanism (to form hemiacetal)
activate the carbonyl with catalytic acid to form a fantastic target
alcohol attacks the slightly positive carbon
proton transfer and form hemiacetal
(to form acetal)
protonate the alcohol to form a good leaving group
2nd alcohol attacks target
proton transfer and form acetal
what biological molecule contains hemiacetals and acetals?
sugars!!! (Ester linkages)
How to convert hemiacetals and acetals into aldehydes/ketones?
Use H+ and water.
What is an imine and how to form one?
An imine is a temporary latching of an amine onto a ketone (reversible)
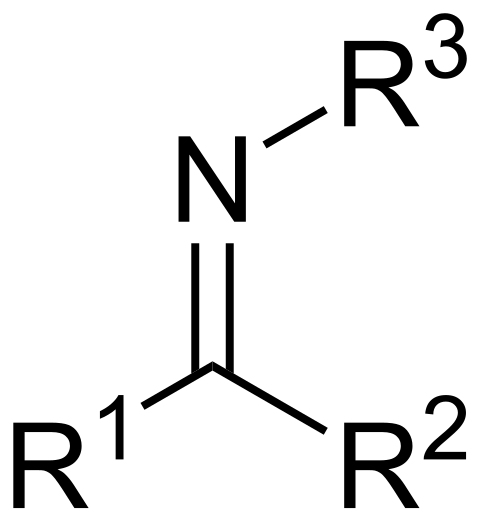
Imines form from the reaction between a 1 prime amine and aldehyde/ketone
what is a enamine and how to form one?
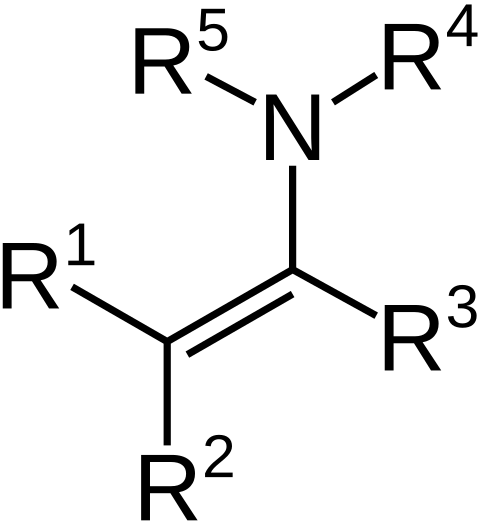
Enamines are formed by reacting a 2 prime amine (two carbon chains on nitrogen) with aldehydes/ketones
What step is necessary to form enamine that does not happen in forming an imine, and why?
An E2-like step is necessary in forming an enamine to neutralize the nitrogen.