Electron sharing and pair-sharing reactions
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
radical
a chemical species that has an unpaired electron
can be described as:
atomic: single atom
polyatomic: group of atoms bonded together with no overall charge
anionic: atom/molecule that gains an electron to become anion and has an atom with an unpaired electron
cationic: atom/molecule that loses an electron to become cation and has an atom with an unpaired electron
reactivity of radicals
unpaired electron makes them highly reactive
high enthalpy
it is energetically favourable for radicals to react and form products with a lower enthalpy, which can be done by:
taking an electron from another species
combining with another radical to form a covalent bond
homolytic fission
homolytic fission is breaking a covalent bond in a way that each atom takes an electron from the bond to form 2 radicals
movement of a pair of electrons is shown using a curly arrow, and a fish hook for one electron
since bond breaking is endothermic, energy is required for homolytic fission
amount of energy needed depends on bonds present:
for weaker bonds, heat is sufficient (thermolytic fission)
for stronger bonds, UV light is necessary (photolytic fission)
halogenation of alkanes
alkanes are very stable and non-polar due to the near identical electronegativities of carbon and hydrogen, so they are very difficult to break
they can undergo free-radical substitution in which a hydrogen atom gets substituted by a halogen
UV light is necessary as alkanes are very unreactive
mechanism
initiation: homolytic fission of halogen
propagation: radicals create further radicals
termination: 2 radicals collide with eachother
propagation step
reactive radicals will attack the unreactive alkanes
a C-H bond breaks homolytically, forming an alkyl free radical
the alkyl free radical is also extremely reactive and can react with a halogen molecule to regenerate another halogen radical, the process can repeat itself
termination step
when 2 radicals react together to form a non-radical product
multiple products are possible
nucleophile Nu:-
electron-rich species that can donate a pair of electrons to form a coordinate bond
all have LP, can be negative or neutral
nucleophilic reaction: nucleophile attacks carbon atom which carries partial positive charge
nucleophile replaces the atom with a partial negative charge
nucleophilic substitution
halogenoalkanes undergo nucleophilic substitution due to the polar C-X bond
nucleophiles form a coordinate bond to the carbon
the bond between carbon and halogen breaks, halogen leaves as the halide ion (leaving group)
the lower the halogen in the group, the faster the rate of reaction/substitution due to decreasing bond enthalpy
heterolytic fission
breaking a covalent bond so that the more electronegative atom takes both the electrons from the bond, forming a negative ion and leaving behind a positive ion
the negative ion is a nucleophile, the positive ion is an electrophile
electrophile E+
species that forms a covalent bond when reacting with a nucleophile by accepting electrons
electron deficient, so will have positive or partial positive charge
e.g H2O, halogens
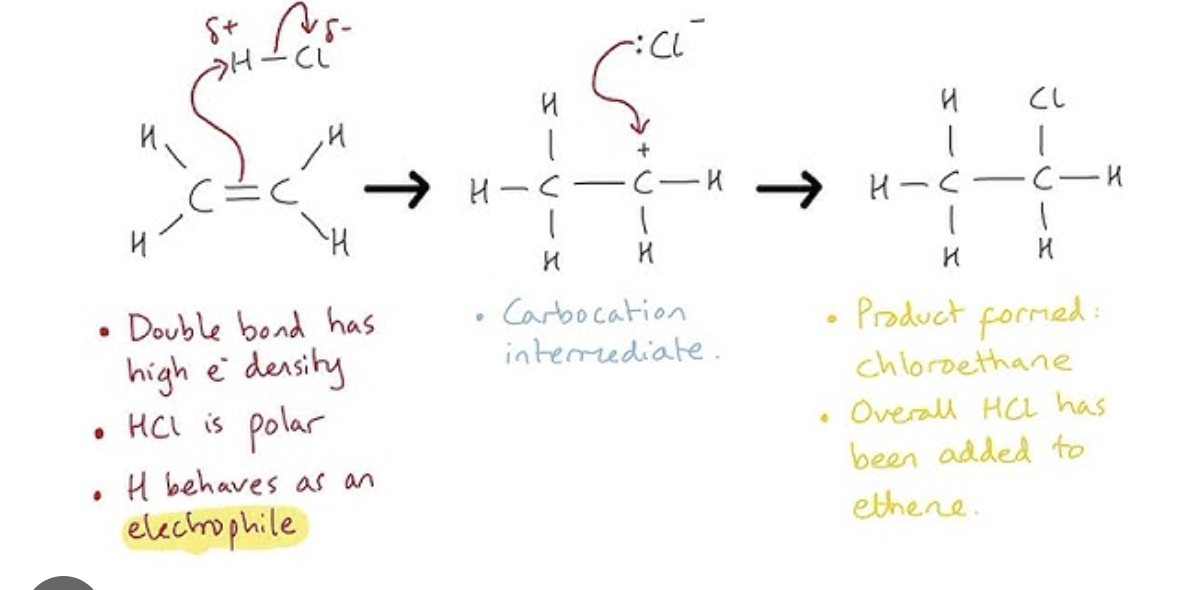
electrophilic addition
addition of an electrophile to an alkene double bond, an area of high electron density
contain pi bonds which are easier to break, meaning alkenes can undergo addition reactions
this makes alkenes much more reactive than alkanes
C=C bond breaks by forming a single bond and 2 new bonds from each C atom
addition of water (hydration)
alkenes treated with steam at 300oC , 60 atm pressure and sulphuric acid catalyst
water is added across double bond, converting alkene into alcohol
C2H4 —→ C2H5OH
water is weak electrophile so doesn’t undergo addition reactions unless in presence of strong acid catalyst
step 1
pi electrons in C=C are attracted to H3O+
heterolytic fission occurs forming carbocation
step 2
water acts as a nucleophile and donates a pair of electrons to the positive carbon atom forming a C-O bond
equilibrium established between positive product and alcohol
addition of halogens (halogenation)
electrophile (halogen) joins onto double bond, results in dihalogenoalkane
halogens can be used to test whether a molecule is unsaturated
unknown compound shaken with bromine water which is yellow, if unsaturated, addition reaction will occur and coloured solution will decolourise
halogens have a temporary dipole caused by repulsion of halogens by the high electron density of the C=C bond
follow same mechanism as in hydrogen halides
addition of hydrogen halides (hydrohalogenation)
hydrogen and halogen added across double bond
due to decreasing bond enthalpy down the group, the fastest reaction is HI
hydrogen halides are polar due to different electronegativities
halogen has a partial negative charge, and hydrogen partial positive
the H atom acts as electrophile and lewis acid by accepting a pair of electron from C=C in alkene
H-X breaks heterolytically forming X- ion
this forms a highly reactive carbocation which reacted with X- ion
polymers
large molecules made from repeating subunits (monomers)
natural polymers: proteins, DNA
synthetic polymers: plastics
addition polymers
many monomers containing at least one C=C double bond form long chains of polymers as the only product
properties of plastics
low weight
polymers loosely packed so less dense and lighter
unreactive
addition polymers from alkenes are saturated and non polar so unreactive
water resistant
polymers are hydrophobic
strong
polymers are made up of many strong covalent bonds
condensation polymerisation
made from monomers containing at least 2 reactive functional groups
polymer is produced by repeated condensation reactions between monomers
involves the elimination of a small molecule (in natural condensation polymerisation this is water)
polyester
polymer formed by condensation polymerisation of dicarboxylic acid and diol monomers
diol: contains 2 OH groups
dicarboxylic acid: contains 2 carboxlic acids -COOH
when polyester is formed, an H atom on one of the diol and an OH group from one of carboxylic acid groups are eliminated as water
polyamides
polymers where repeating units are bonded together by amide links
amide group: -CONH
diamine and dicarboxylic acid required
OH from COOH and H from NH2 expelled as water molecule forming amide link
biodegradable polymers
polyesters and polyamides can be broken down using hydrolysis, making them better for the environment than addition polymers
lewis acids and bases
lewis acid: lone pair acceptor
lewis base: lone pair donor
bronsted-lowry vs lewis
bronsted-lowry acid: species that can donate H+
lewis acids cover a broader spectrum, they can accept a lone pair of electrons which includes H+. bronsted-lowry considers acids as H+ donors only
bronsted-lowry base: species that can accept H+
formation of coordination bonds
when NH3 and BF3 react, coordinate bond is formed
occurs as lone pair on nitrogen atom can be donated to boron (electron deficient) creating NH3BF3
NH3 acts as lewis base (nucleophile) and BF3 as lewis acid (electrophile)
since only electrons are being donated/accepted, neither is a bronsted-lowry acid/base
lewis acids/bases in complex ions
complex ion: a central transition mental ion surrounded by ligands bonded to it by coordinate bonds
ligands are lewis bases, so must have a lone pair and/or a negative charge
transition metal acts as the electrophile (lewis acid) and ligands act as nucleophiles (lewis bases)
different ligands form different numbers of coordination bonds to central metal ion
monodentate
bidentate
polydentate
coordination number: number of coordinate bonds to central metal
can be same as the number of ligands if they are monodentate
representing complex ions
square brackets used to group together ligands and metal ion
overall charge is the sum of oxidation states of all species present
complexes with coordination number of 4 are usually tetrahedral, coordination number 6 usually octahedral
bidentate ligands
each form 2 co-ordinate bonds to central metal ion
because each ligand contains 2 atoms with lone pairs
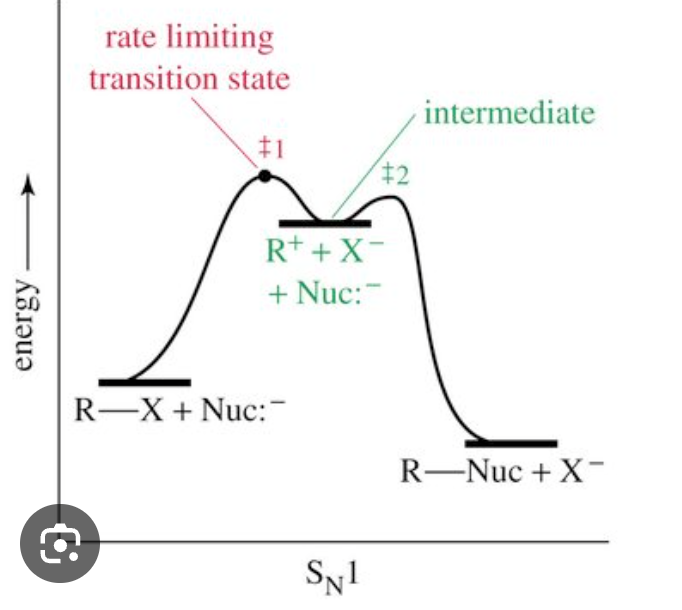
Sn1 reactions
nucleophilic substitution
occurs in tertiary halogenoalkanes (carbon bonded to halogen is bonded to 3 alkyl groups)
1 = the rate of reaction (determined by slowest step) depends on conc of halogenoalkane
Sn1 mechanism
2 step reaction
1st step
C-X bond breaks heterolytically, halogen leaves as an X- ion (this is the slow step)
forms tertiary carbocation intermediate (tertiary carbon atom with positive charge)
2nd step
tertiary carbocation is attacked by nucleophile
Sn2 reaction
nucleophilic substituion
occurs in primary halogenoalkanes (carbon bonded to halogen bonded to 1 alkyl group)
2 means rate is dependent on conc of halogenoalkane and nucleophile ions
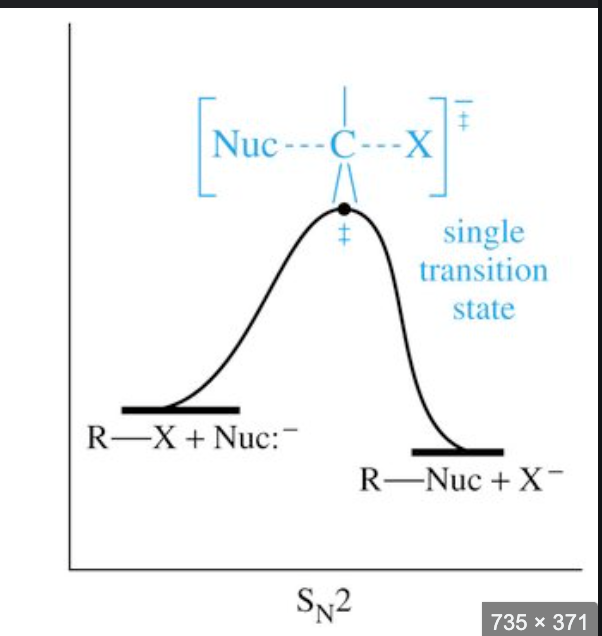
Sn2 mechanism
1 step
nucleophile donates a pair of electrons to partially position C atom forming a new bond
at the same time, C-X bond breaks by heterolytic fission and halogen leaves as X-
sometimes the halogen atom in halogenoalkane causes steric hinderance
the nucleophile can only attack from the opposite side of the C-X bond
as a result, the molecule undergoes an inversion of configuration
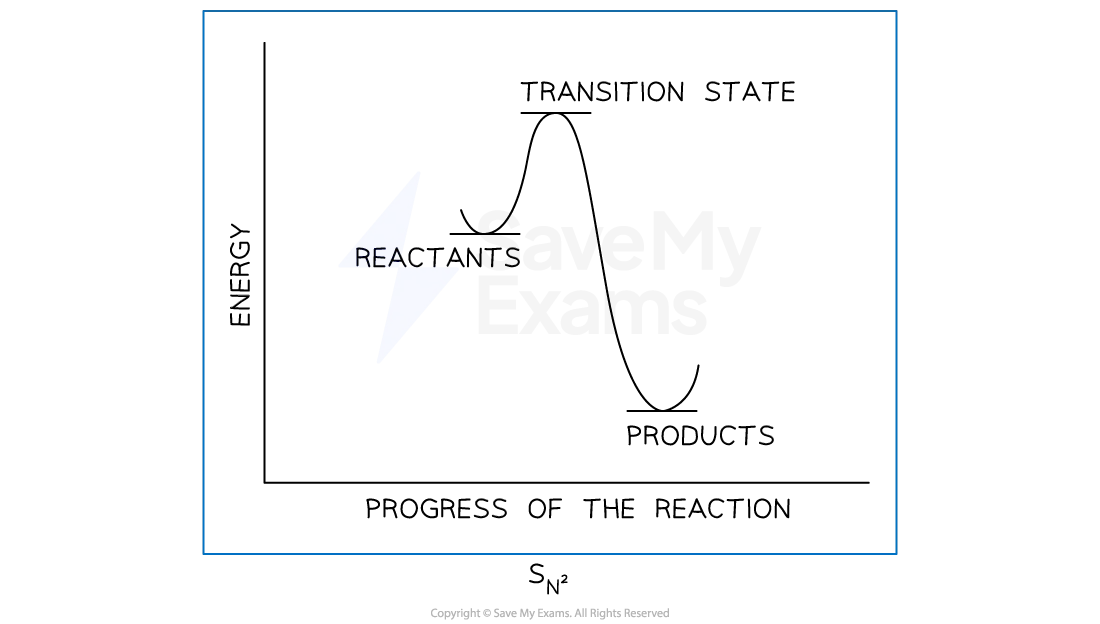
what influences rates of nucleophilic substitution
the nature of nucleophile
the halogen involved
the structure of the halogenoalkane
the nature of the nucleophile
the greater the electron density on nucleophile, the stronger the nucleophile
negative anions tend to be more reactive than their corresponding neutral species e.g OH- and water molecules
when nucleophiles carry the same charge, electronegativity of atom carrying the lone pair becomes deciding factor
the less electronegative the atom carrying the lone pair, the stronger the nucleophile
this is because a less electronegative atom has a weaker grip on its lone pair
the halogen involved
substitution reactions break the C-X bond, so bond energies can be used to explain their different reactivities
so F>Cl>Br>I in order of most to least strong
thus I has the fastest rate
the structure of the halogenoalkane
tertiary halogenoalkanes undergo Sn1, forming stable tertiary carbocations
fastest
secondary halogenoalkanes undergo a mix of both Sn1 and Sn2 reactions depending on structure
primary halogenoalkanes undergo Sn2 reactions forming less stable primary carbocations
slowest bc involves a high activation energy transition state
asymmetric alkenes
contain different groups attached to carbon atoms of the C=C bond
markovnikov’s rule
when hydrogen halides add to asymmetric alkenes, 2 products are possible depending on which C atom the H atom bonds to
markovnikov’s rule predicts which isomer will be the major product
the H atom will add to the C atom that already contains the most H atoms bonded to it
explanation of markovnikov’s rule
stability of intermediate carbocation must be considered
alkyl groups have a positive inductive effect (they push electron density away towards the positive charge density of the carbocation, which partially stabilises the charge)
when charged carbon surrounded by 1 alkyl group it is primary, 2 second, 3 tertiary
the more alkyl groups, the more stable the carbocation, the more likely the reaction mechanism goes by that intermediate
electrophilic substitution in benzene
nitration: substitution of a hydrogen atom from the benzene ring with an electrophile
benzene combined with nitric acid and catalysed w/sulphuric acid at 50 Celsius
step 1: generation of electrophile
delocalised pi system is extremely stable and a region of high electron density
electrophile for nitration is nitronium NO2+
NO2+ is attracted to delocalised pi electrons
step 2: electrophilic attack
pair of electrons from benzene ring is donated to electrophile and to form covalent bond
bond is formed between carbon atom and electrophile forming carbocation intermediate
disrupts aromaticity in ring
step 3: regenerating aromaticity
C-H bond breaks and leads to reforming of benzene ring, electrons in bond go to benzene ring
products: nitrobenzene and H+
H+ combined with H2SO4- to reform catalyst