13. reoviridae & general arboviruses
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
steps in rabies pathogenesis
entry = skin via bite wound
local replication
can be blocked by antibodies via vaccination pre- or post-exposure
spread via nerves → NO viremia
replicate in brain neurons
spread via nerves to salivary glands
shed in saliva
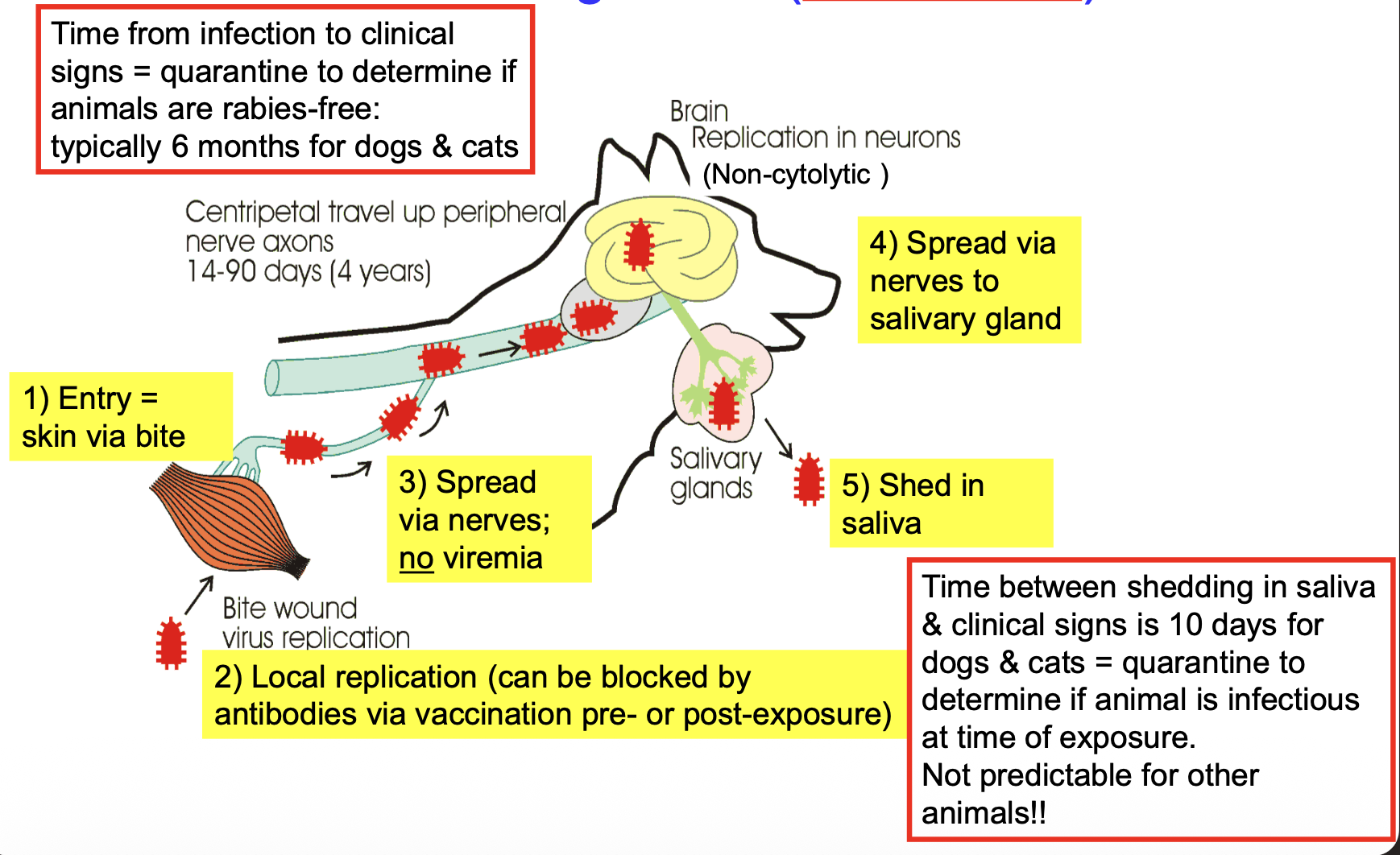
in dogs and cats, what is the duration from rabies infection to clinical signs?
typically 6 months → quarantine to determine if animals are rabies-free
in dogs and cats, how long is the time between shedding in saliva & clinical signs? can this duration be used in other animals?
10 days → quarantine (after bite) to determine if animal was infectious at time of exposure
NOT predictable for other animals
what type of genome do reoviridae viruses have?
segmented RNA → reassortment can occur
are reoviridae naked or enveloped?
naked → very stable in environment
what 3 reoviridae genera have veterinary importance?
orthoreoviruses = respiratory, enteric, systemic disease
rotaviruses = enteritis in young
orbiviruses = arboviruses → systemic disease
rotaviruses are a very important cause of what pathologic condition? what age group is most severely affected by rotavirus spp.?
diarrhea in young animals
usually < 2 months
most severe in neonates
rotavirus host range
many species, including humans, foals, calves, lambs, piglets, rabbits, etc.
many serotypes — host specific
rotavirus transmission
fecal-oral
virus shed at high levels in feces
very stable in environment
rotavirus pathogenesis
from lumen, rotavirus infects epithelial cells at the tips of the villi in small intestine → villous atrophy → malabsorption diarrhea
reduced levels of lactase in gut & impaired glucose-dependent sodium transport → osmotic/maldigestion diarrhea
undigested lactose promotes secondary bacterial infection
viral protein (NSP4) acts as enterotoxin → secretory diarrhea
rotavirus clinical signs
1-24 hour incubation period → rapid onset
voluminous soft/liquid diarrhea ± mucus
“milk or white scours”
distended abdomen
severe diarrhea → dehydration → possible death
what makes an arthropod a biologic vector?
virus replicates in the arthropod & is secreted in saliva when it feeds on new host
arboviruses
what are examples of virus families that contain arboviruses? (from handout)
togaviridae
flaviviridae
bunyaviridae
reoviridae
rhabdoviridae
asfarviridae
what makes an arthropod a mechanical vector?
virus does not replicate in the arthropod & is transmitted on arthropod’s mouth parts during arthropod biting & feeding
examples of viruses that are transmitted via mechanical vectors
swinepox virus
myxoma virus
equine infectious anemia virus
lumpyskin disease virus
what is an enzootic cycle?
transmission that maintains the virus in nature
involves amplifying host and arthropod vector
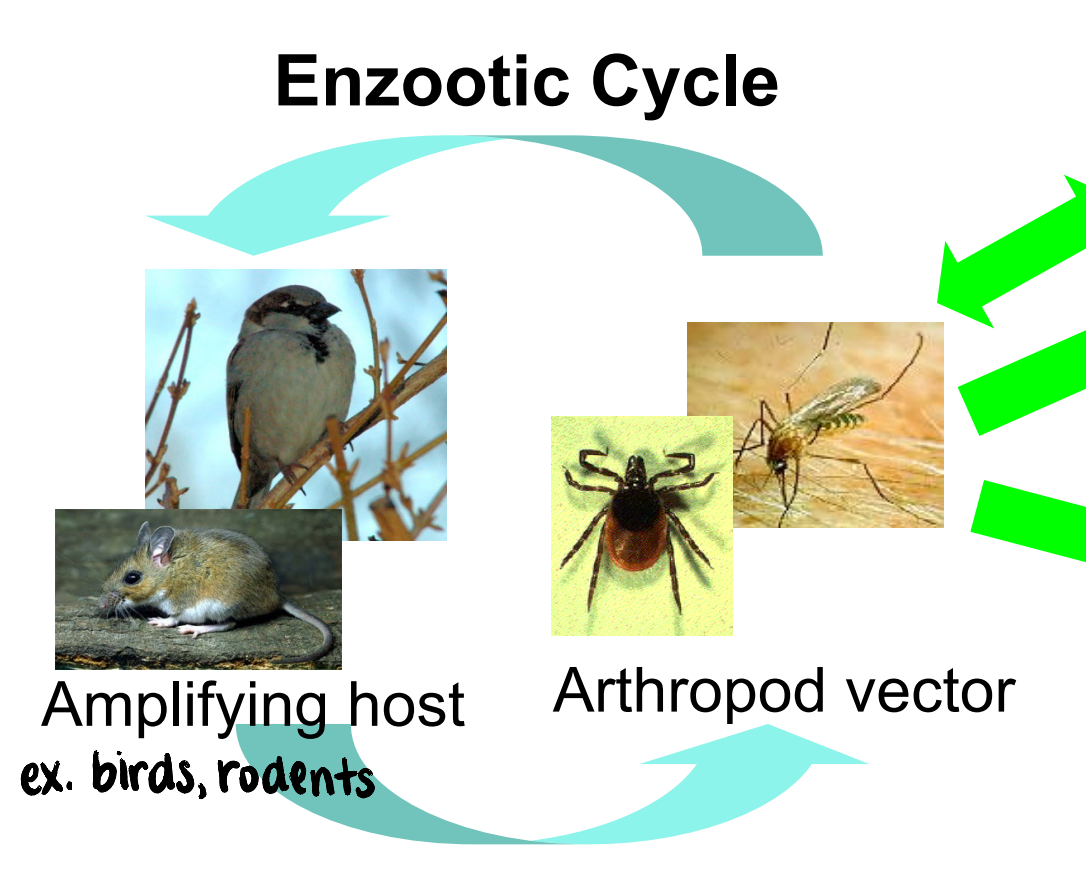
what is an epizootic cycle?
transmission that results in disease outbreak
often due to “spill-over” into dead-end hosts
virus itself, amplifying host, and mosquito species may differ from the enzootic cycle
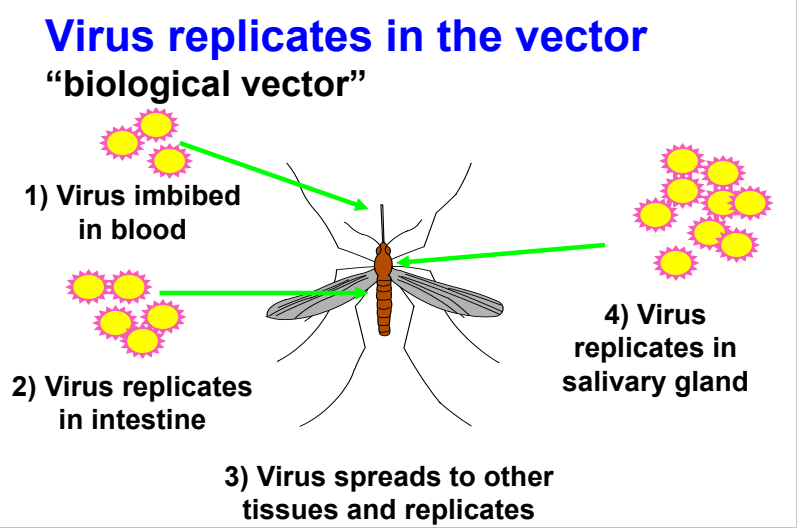
steps in viral replication within biological vector
virus imbibed in blood
virus replicates in intestine
virus spreads to other tissues and replicates
virus replicates in salivary gland
steps in viral replication in amplifying vertebrate host
virus inoculated by mosquito bite
virus replicates in tissues
virus in blood
virus transmitted to naive mosquito in blood
amplifies the virus

virus infection in incidental or dead-end host
virus inoculated by mosquito bite
virus replicates in tissues
little or no virus in blood (not enough to infect naive arthropod vector)
important examples of orbiviruses
bluetongue virus
epizootic hemorrhagic disease virus
african horse sickness virus (exotic to US)
geographical distribution of bluetongue virus
worldwide distribution
emerging into new regions of US
spreading in Europe
bluetongue virus vector
culicoides spp. (biting midges)
bluetongue virus transmission cycle
enzootic cycle
amplifying hosts: ruminants
arthropod vector: culicoides spp.

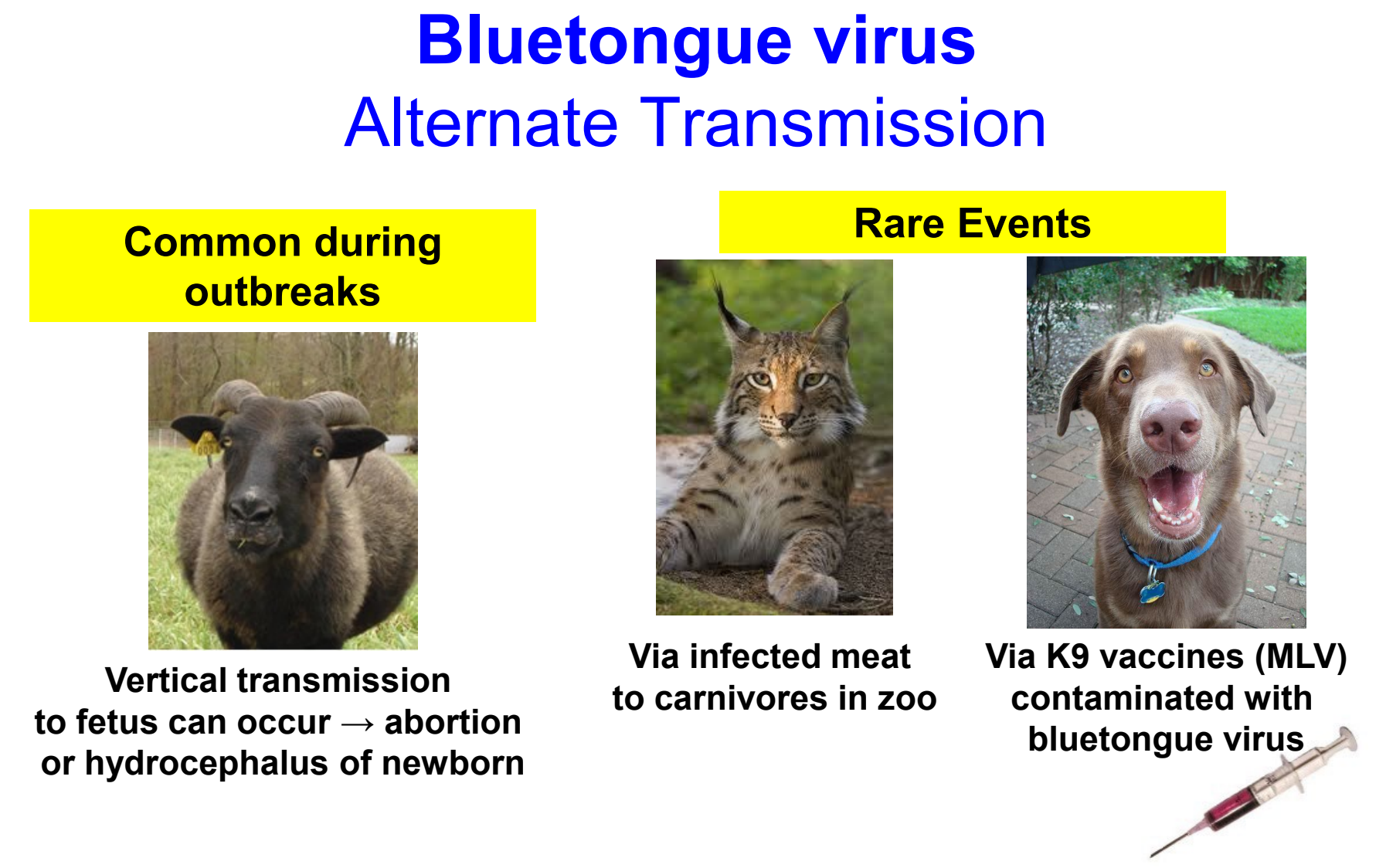
bluetongue virus alternate transmission
vertical transmission to fetus can occur → abortion or hydrocephalus of newborn (common during outbreaks)
rare events:
via infected meat to carnivores in zoo
via contaminated canine vaccines
orbivirus pathogenesis
virus deposited in skin by biting midge
spread to draining lymph node → viremia
replication in macrophages, dendritic cells, and endothelial cells
cytokine response & endothelial cell damage → vascular leakage, hemorrhage, infarctions
what are the 4 “E’s” (clinical signs) that can be observed during orbivirus infection?
erythema of skin and mucosa
erosions of mucosa, skin, teats
edema, especially of head & neck
effusions (pleural & pericardial)
bluetongue disease affects what species?
sheep primarily
also deer, pronghorn antelope, and bighorn sheep
usually subclinical in cattle (but can cause clinical disease)
rare disease in carnivores
NOT zoonotic
clinical signs of bluetongue disease (in sheep)
“sore muzzle disease”
erythema, edema (can rarely cause cyanosis or “blue tongue”), erosions, effusion
coronitis → lameness
abortion or congenital defects (e.g. “dummy lamb” due to hydrocephaly")
hyperemia of skin → “wool breaks” in survivors
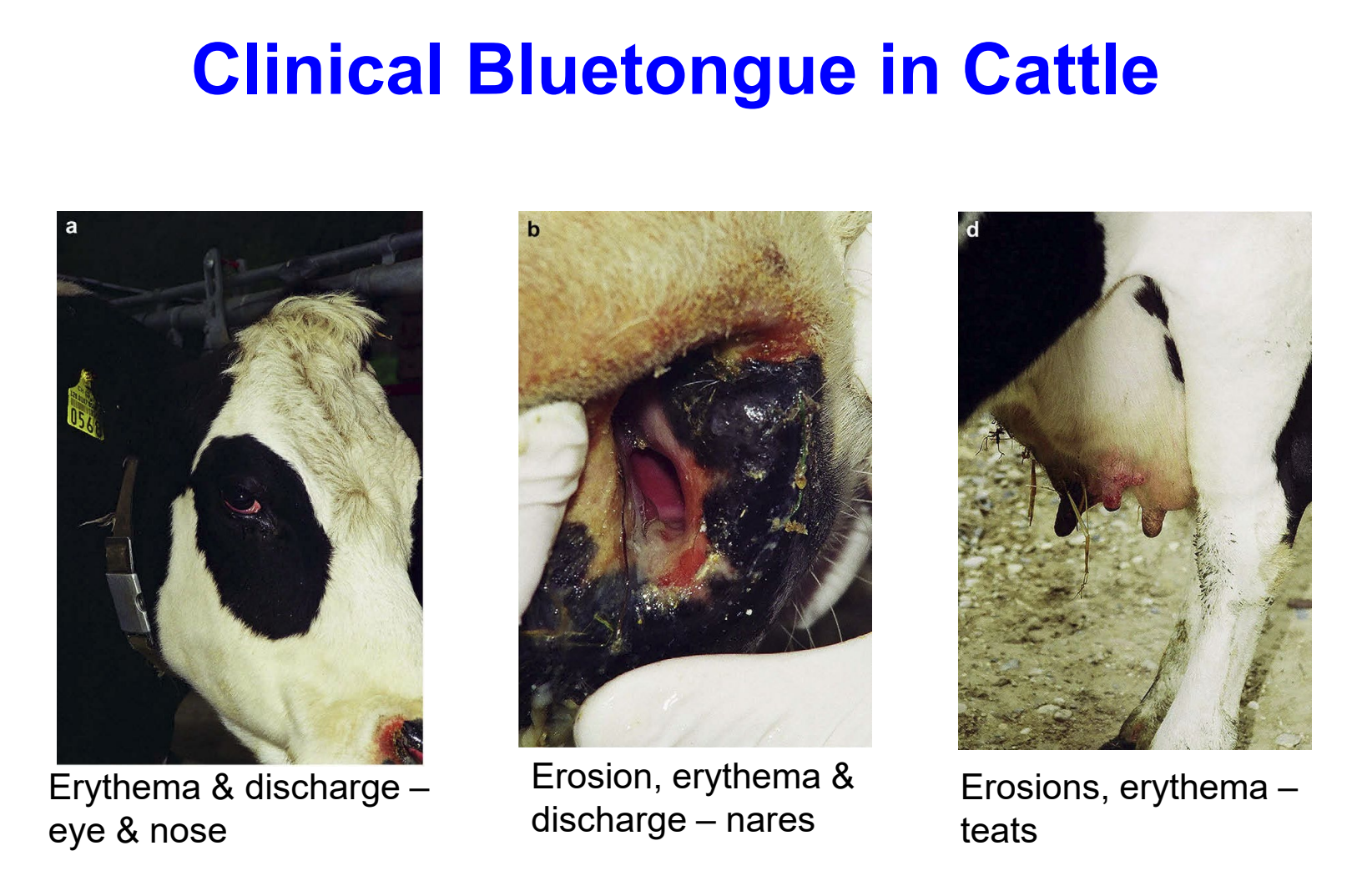
clinical bluetongue in cattle
erythema & discharge — eye & nose
erosion, erythema & discharge — nares
erosions, erythema — teats
bluetongue virus control
vector control
immunity is serotype-specific
vaccine used in areas with disease
import restrictions to prevent introduction of exotic serotypes
reportable in wisconsin
are animals with bluetongue virus directly contagious?
NO → no shedding from lesions or mucosal membranes
epizootic hemorrhagic disease (EHD) is one of the most important diseases of what species?
white-tailed deer
very similar to bluetongue disease
geographical distribution of EHD
disease emerging into new regions of US with some disease in cattle
disease outbreaks common
annual outbreaks in southeastern US
sporadic outbreaks in other regions
epizootic hemorrhagic disease vector
culicoides spp. (biting midges)
EHD host range
primarily disease of white-tailed deer
NOT sheep
disease in cattle can occur (during outbreaks in deer)
NOT zoonotic
EHD important clinical signs in deer
erosions in mouth
disseminated intravascular coagulation (DIC) → not usually seen with BTV
mortality ~33%

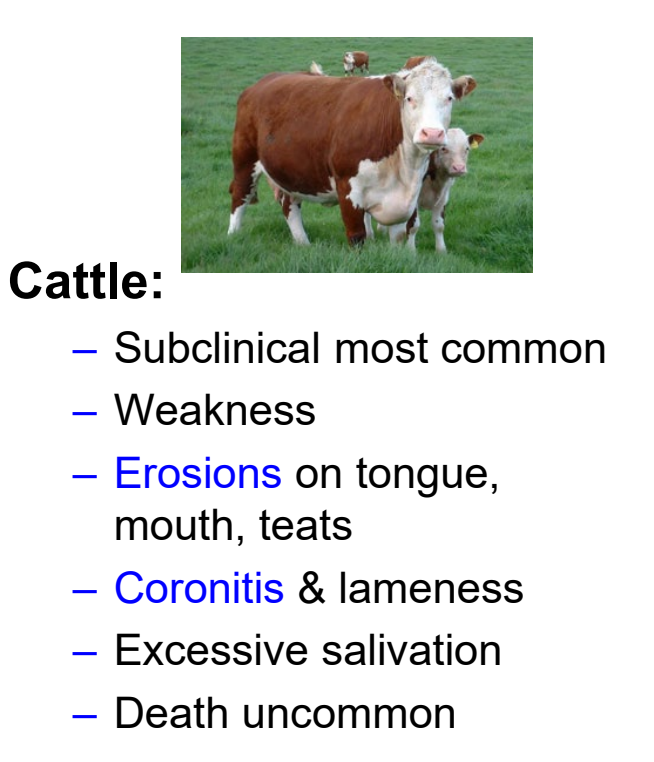
EHD important clinical signs in cattle
subclinical disease most common
erosions on tongue, mouth, and teats
coronitis & lameness
death uncommon
EHD control
vector control
reportable in wisconsin
erosion
destruction of surface layer of skin (epidermis) or mucosa (epithelium)
basement membrane is intact
ulcer
destruction into deeper layers of skin (dermis) or mucosa (submucosa)
deeper than basement membrane
vesicle
fluid filled sac within skin epidermis or mucosal epithelium
blister-like
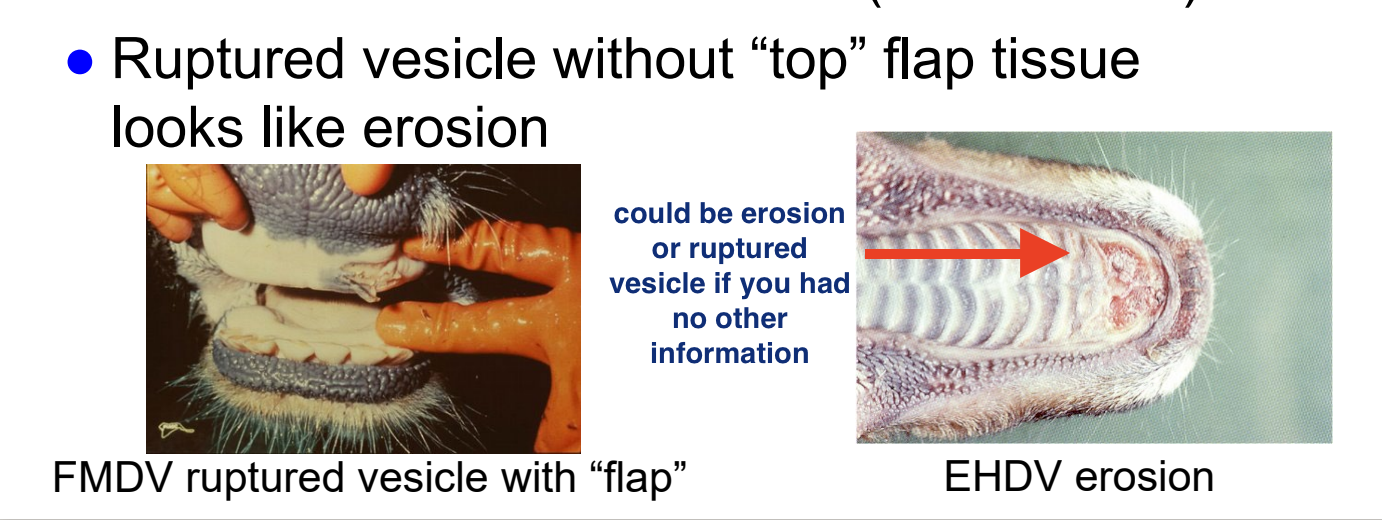
what is an important thing to consider when evaluating BTV/EHDV lesions?
BTV and EHD cause erosions in mouth and coronary band
in ruminants, must R/O foot-and-mouth disease (vesicular lesions; foreign animal disease)
ruptured vesicle without “top” flap of tissue looks like erosion
why would you not want to use a mucosal tissue sample when diagnosing bluetongue virus/EHD?
virus does not replicate in mucosa → infarction/lack of blood supply secondary to severe edema kills the mucosa, NOT viral replication
african horse sickness (AHS) geographical distribution
**EXOTIC to US**
enzootic in sub-saharan Africa
outbreaks in europe, middle east
african horse sickness vector
culicoides spp. (biting midges)
how does african horse sickness compare to bluetongue virus?
pathogenesis is very similar to bluetongue virus except no erosions observed
african horse sickness host range
primarily in equines
horse > mule > donkey » zebra
zebras are important reservoir
fatal disease in dogs can occur (eating infected horse meat)
NOT zoonotic
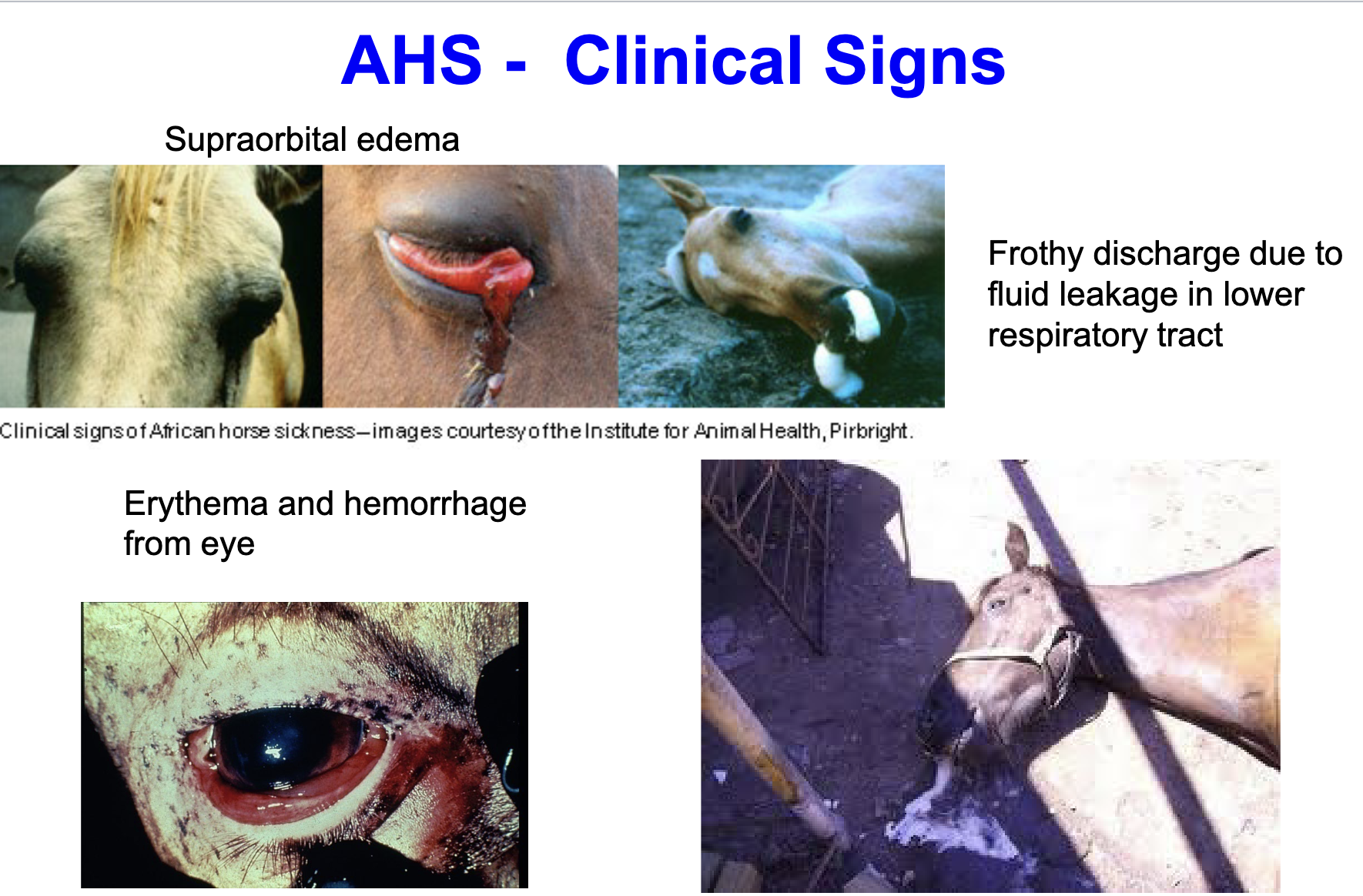
AHS major clinical signs
supraorbital edema
frothy discharge due to fluid leakage in lower respiratory tract (pulmonary form)
erythema and hemorrhage from eye
high mortality (50-95% in horses)
AHS gross pathology
pulmonary edema
froth in airways
pleural & pericardial effusions
petechial hemorrhages
is AHS reportable?
YES
exotic disease
reportable within 1 day
AHS control
surveillance/quarantine for horses imported from enzootic regions
vector control
vaccines in enzootic regions
no cross protection between serotypes