Stereochemistry
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
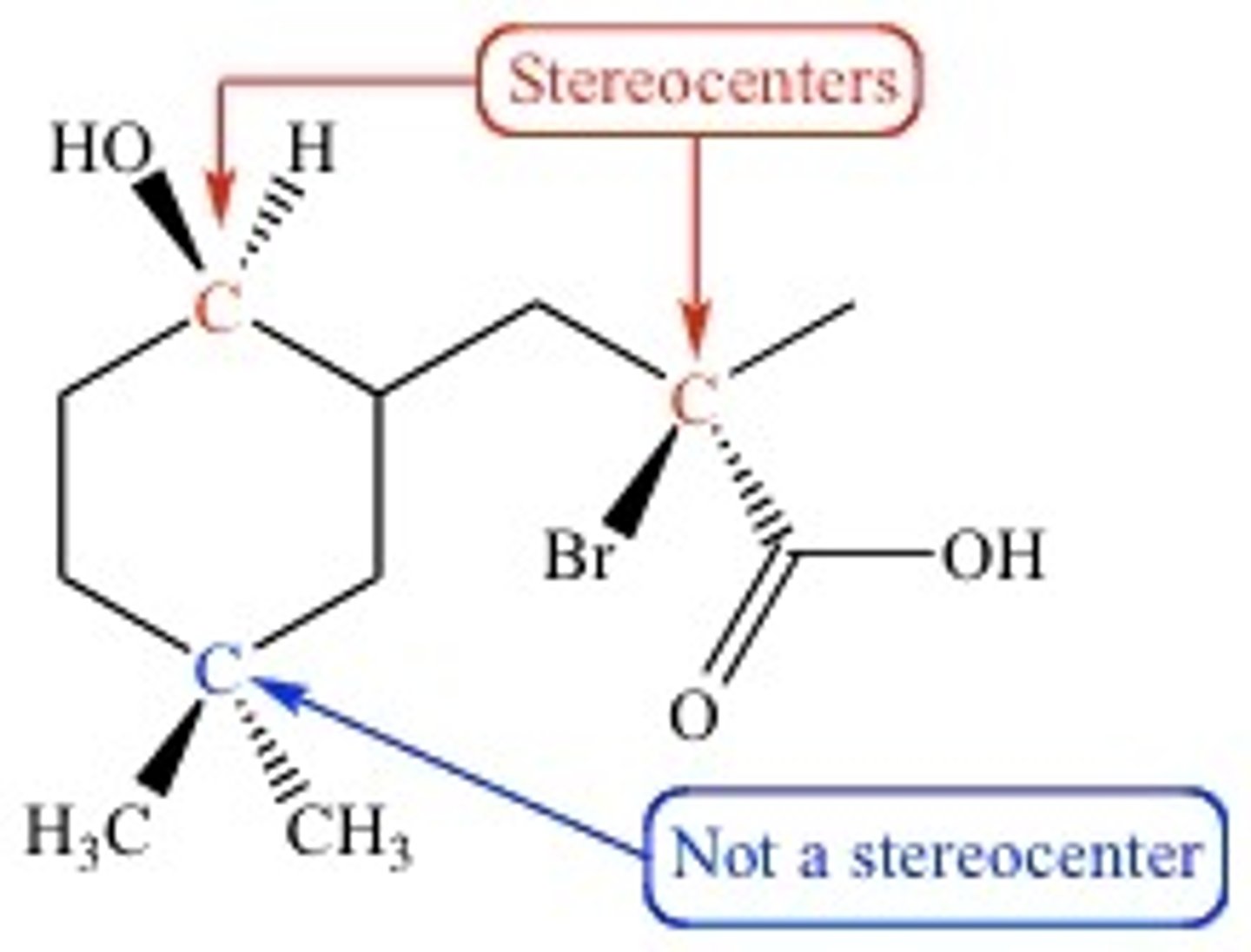
chiral center
tetrahedral atom with four different substituents
lacks a plane of symmetry
achiral molecule
rotated molecule can be superimposed on its mirror image
has a plane of symmetry
chiral molecule
a molecule that has a chiral center and lacks a plane of symmetry
rotates polarized light
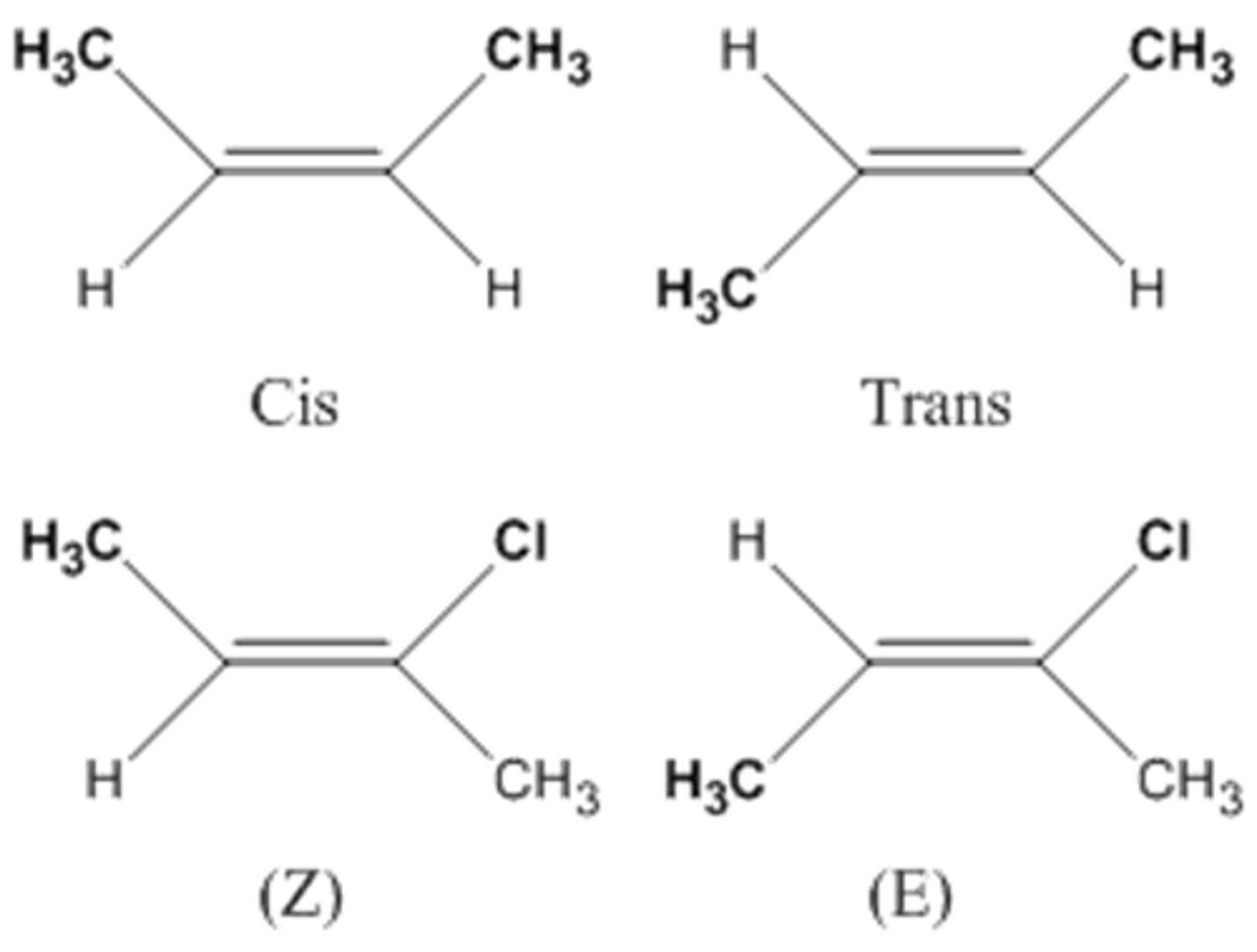
geometric isomers
Compounds that have the same molecular formula but differ in the spatial arrangements of their atoms.
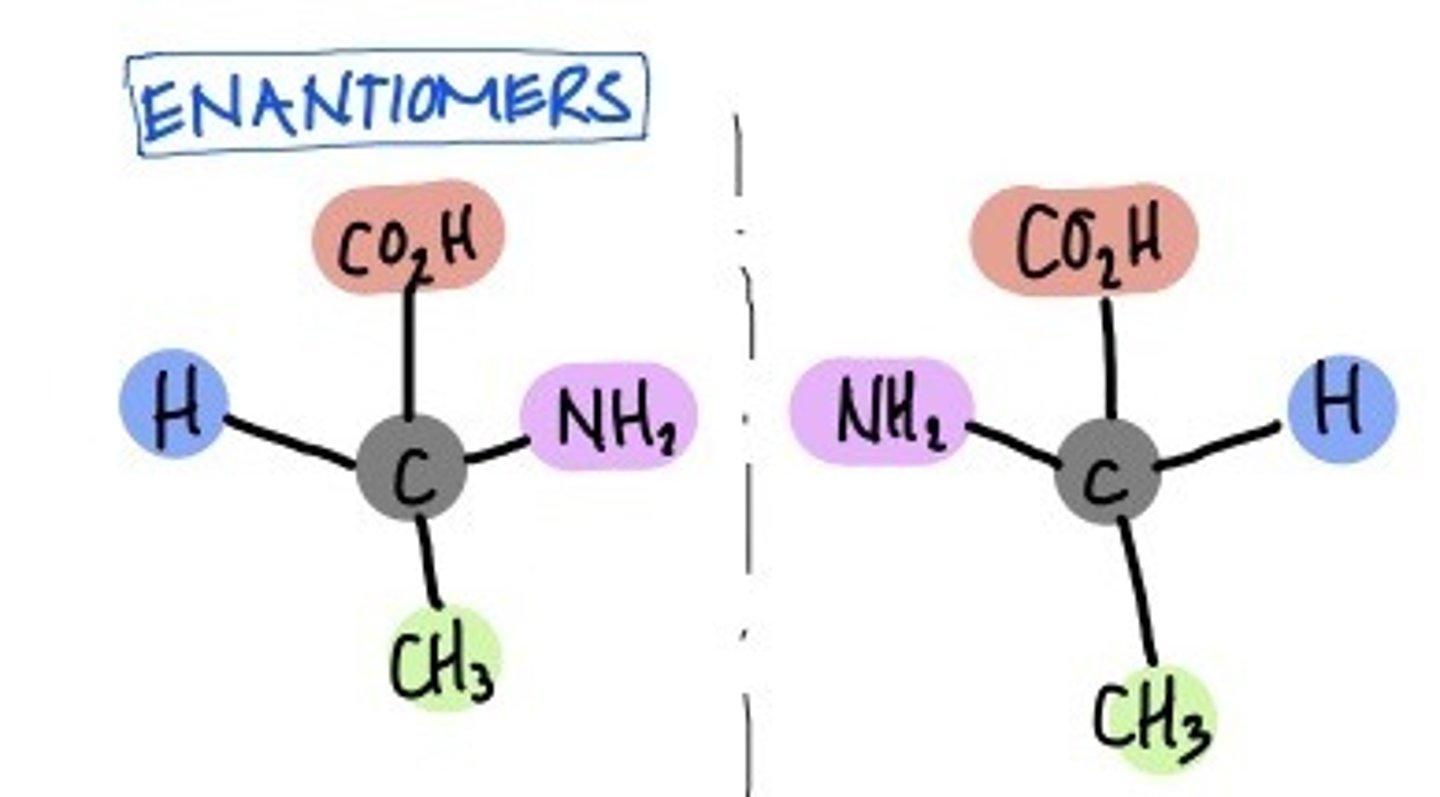
Enantiomers
isomers that are mirror images of each other but are not superimposable
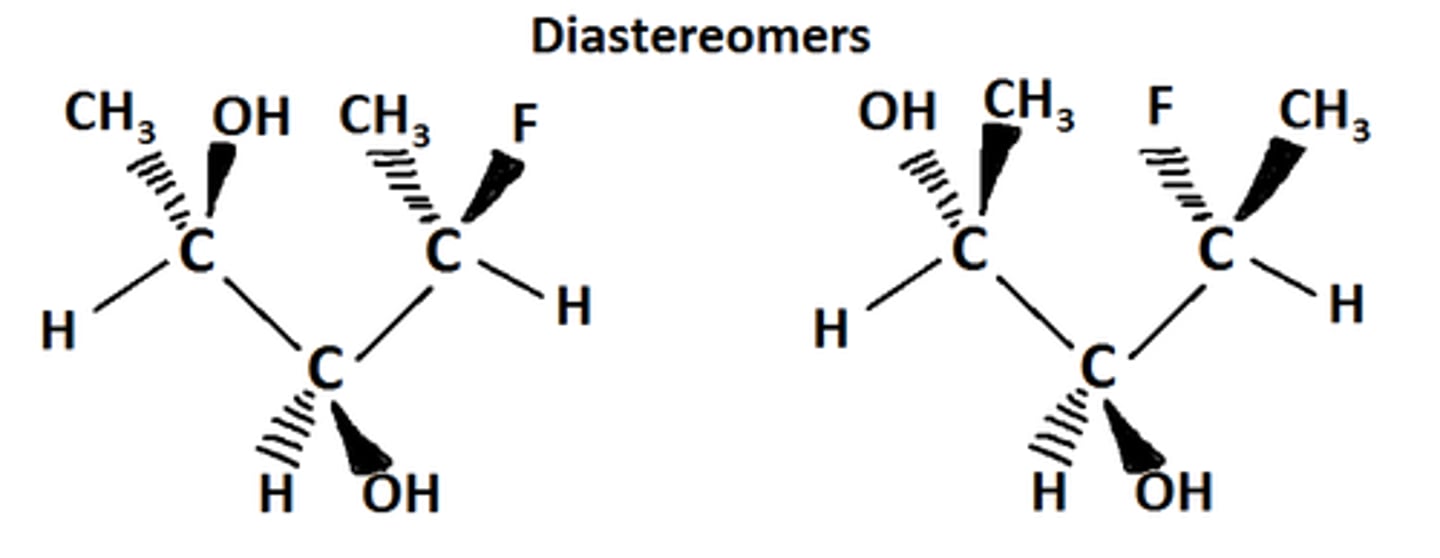
Diastereomers
stereoisomers that are not mirror images of each other
must have at least 2 stereo centers
Priority ranking in determining chirality
Based on atomic number
Ex: F > nitro > carboxyl > propyl
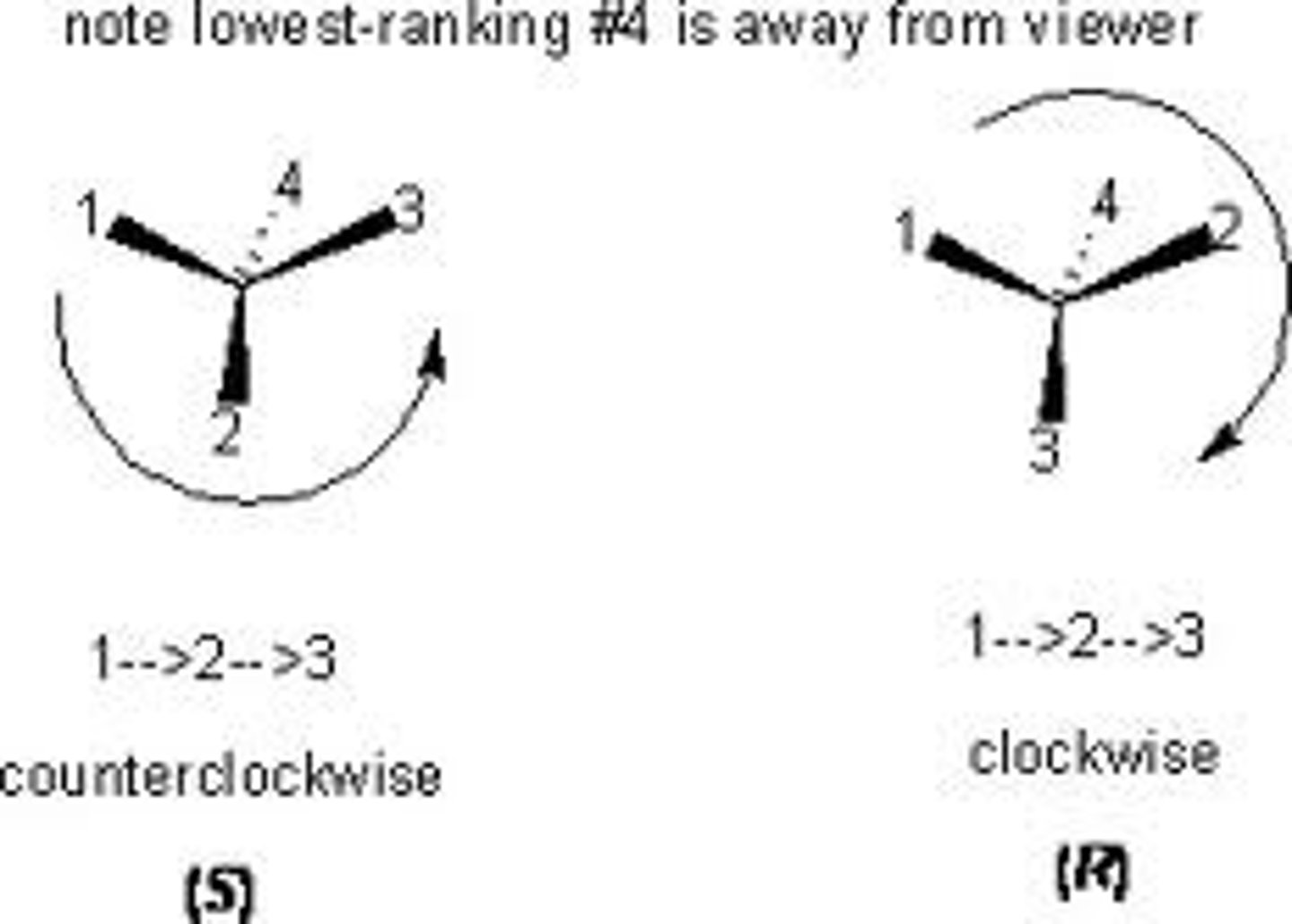
R and S
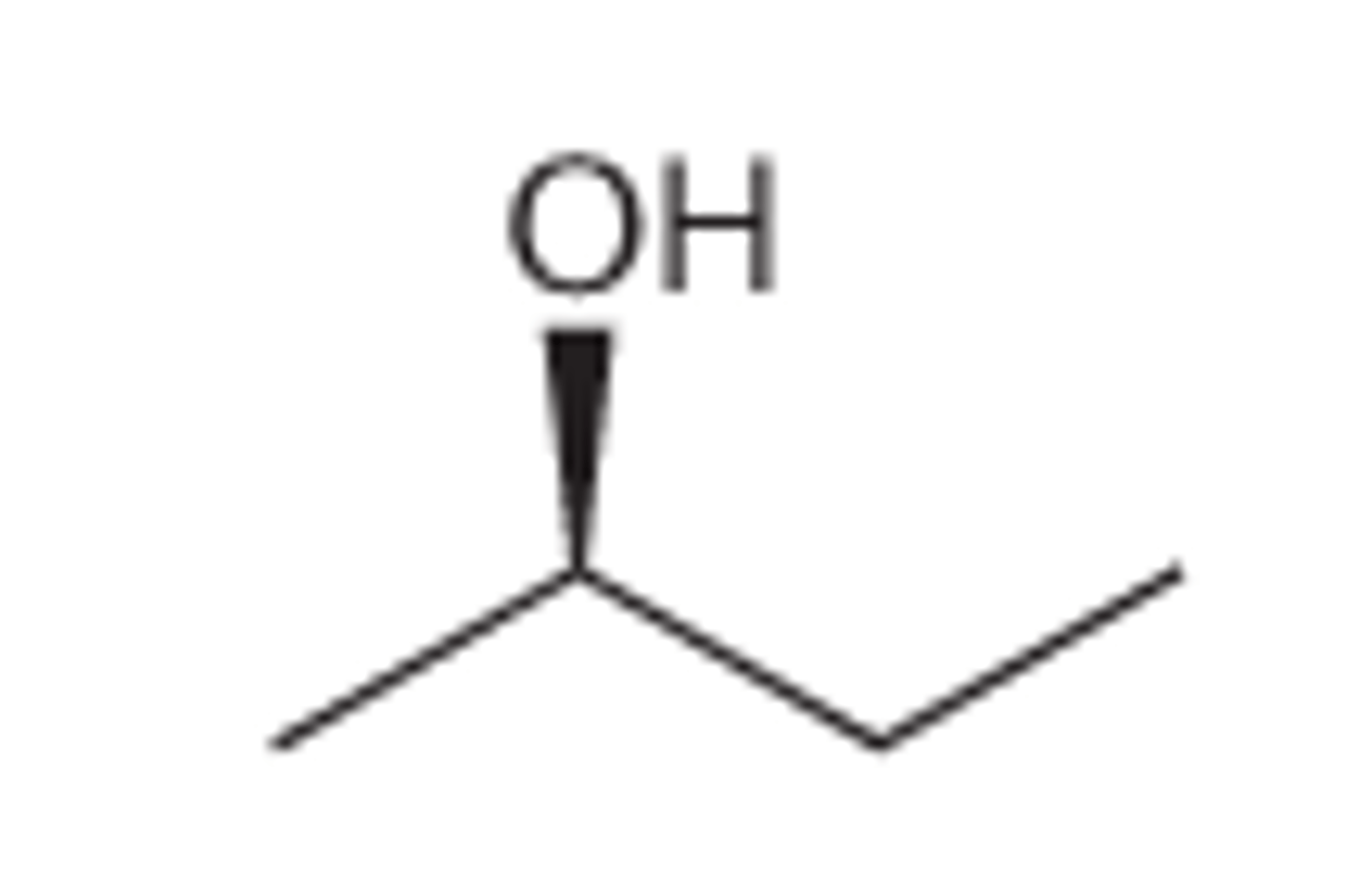
Draw (R) 2-butanol, CH3CHOHCH2CH3
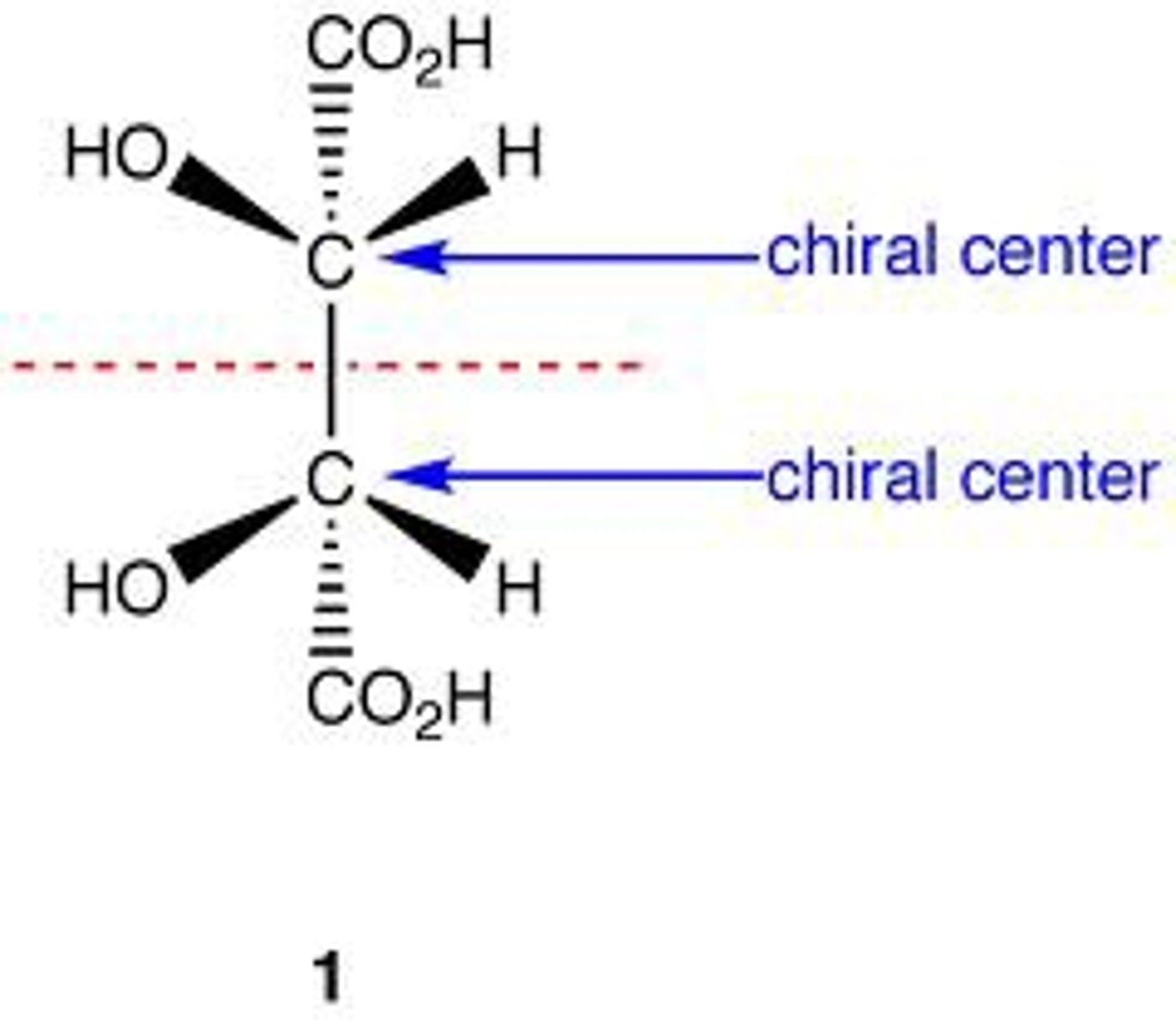
meso
an achiral compound that contains chiral centers
RS and SR
have a plane of symmetry between the chiral centers
optical activity
rotation of the plane of polarized light
optical activity of enantiomers
enantiomers have equal and opposite specific rotations
racemic mixture
A mixture that contains equal amounts of the (+) and (-) enantiomers.
Racemic mixtures are not optically active.
optical activity of diastereomers
diastereomers have different specific rotations
optical activity of meso compounds
meso are not optically active
enzymes bind to [ either, both, or just one] chiral molecule
one of the stereoisomers fits the active site of the enzyme
physical properties of enantiomers
identical
physical properties of diastereomers
different
If you are trying to determine R or S, and the H happens to be pointing toward you, then....
You need to either
1. Turn the molecule over and then look at the priorities
OR
2. Assign the priorities and know that your answer is the opposite