Unit 8 Titration Curves
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
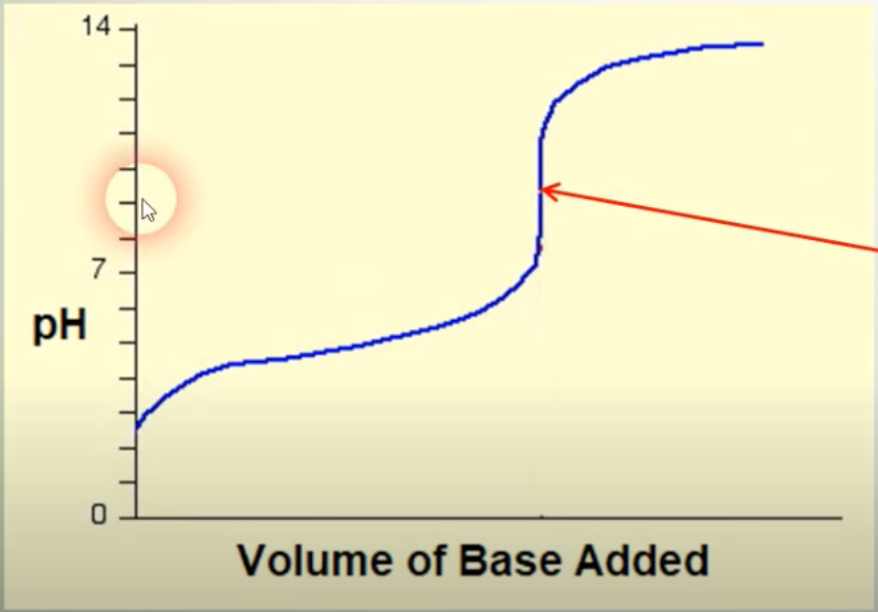
?
equivilance point(molA=molB; only salt + H2O)
2
New cards
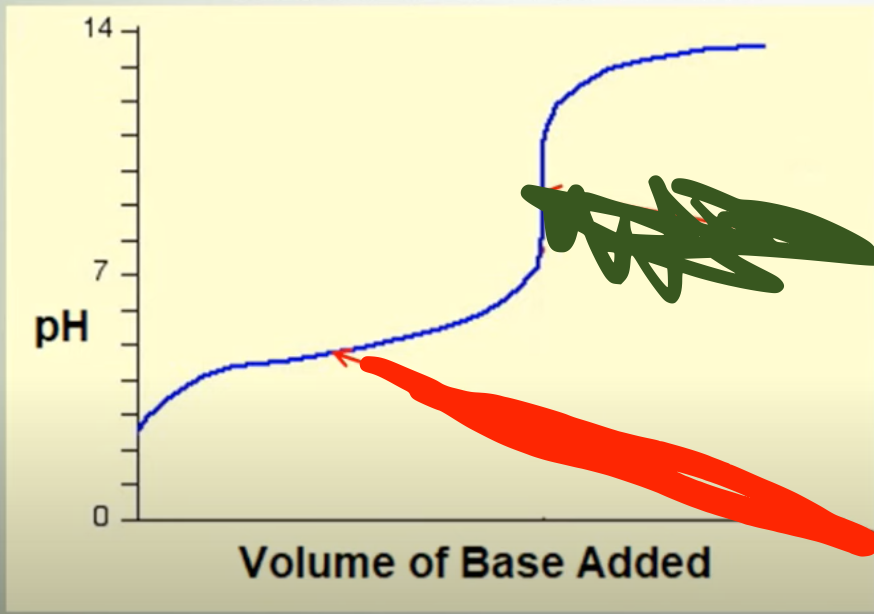
?
½ neutral (WA/SB → pH=pKa)
3
New cards
½ neutral…
WA/SB → pH=pKa →[A-]=[HA] SAME FOR poH=pKb
4
New cards
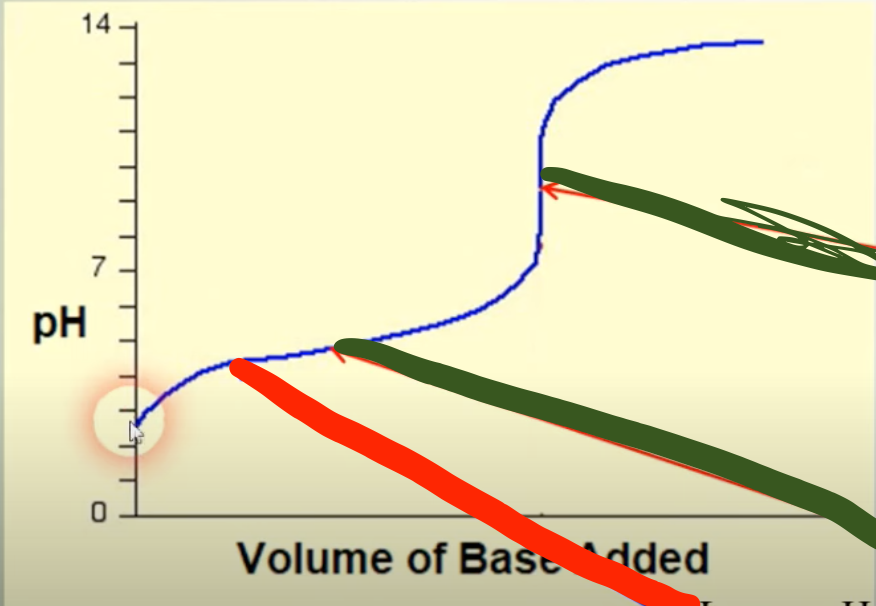
?
5
New cards
SA/SB =
pH=7
6
New cards
pKb goes up =
Kb goes down