HW2_exam prep
1/183
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
184 Terms
Which antibody is most likely to cause a delayed hemolytic transfusion reaction?
anti-Jk^a
As defined by the FDA, all poly-specific antihuman globulin reagents must con- tain _
IgG and C3d
What should be used to confirm all neg- ative anti-globulin tests?
Coombs control cells
The fetal screen performed on a patient was positive. What should be done next?
Perform a quantitative tests such as the Kleihauer-Betke test
A fetomaternal hemorrhage of 65 ml of feta blood has been detected in an Rh negative woman. How many vials of RhIG should be give?
3
If a patient has a negative antibody screen, which of the following may cause a positive result in the major cross- match?
-The presence of an alloantibody against a low frequency antigen on the donor cells
-Prior sensitization of donor red cells -Incorrect determination of the patient's ABO group
All
A group O Rh negative patient has anti-D and anti-K in his serum. Approximately how many units of O negative blood from the Caucasian population would need to be screened to find 3 units of compatible blood?
4
Which of the following situations is most likely to cause mix field agglutination at the AHG phase of the crossmatch?
- performing a crossmatch on a recently transfused patient
- performing a crossmatch on a pregnant woman
- performing a crossmatch on a patient who has anti-Sd^a in his serum
- performing a crossmatch on a neonate
performing a crossmatch on a patient who has anti-Sda in his serum
According to AABB standards, compati- bility testing must include
test for unexpected antibodies reactive at 37c
An order is received for 6 units of blood on a trauma patient. The antibody screen is negative, and 5 of 6 donor units are compatible using the AHG crossmatch. The incompatible unit is 2+ positive. What is the first step you would take to resolve this problem?
Select and crossmatch additional units/Perform a direct antiglobulin test on the incompatible unit.
Four units were requested on a patient scheduled for cardiovascular surgery to- morrow. The antibody screen is nega- tive at immediate spin through the AHG phase. Three of the four units are com- patible; however, donor unit #1 was 4+ incompatible at immediate spin. What is the most likely cause of the incompatibil- ity?
Tech error in selecting units, and donor unit #1 is ABO incompatible with patient.
Four units of blood were ordered on a lymphoma patient suffering from anemia. The patient has never been transfused and was typed as O negative. The anti- body screen is 2+ positive with all three screening cells and all four units at the AHG phase. The DAT is 3+. What would you do to find compatible blood?
Use the patient serum adsorbed with pa- tient cells at 4c. Elute the antibody from patient cells and use the cells to adsorb the patient serum at 37c.
If a patient in the emergency room ur- gently needs blood and the transfusion service has not had time to determine the patient's ABO group, what should they do?
Issue only group O Rh negative packed cells
results obtained during an antibody screen were:
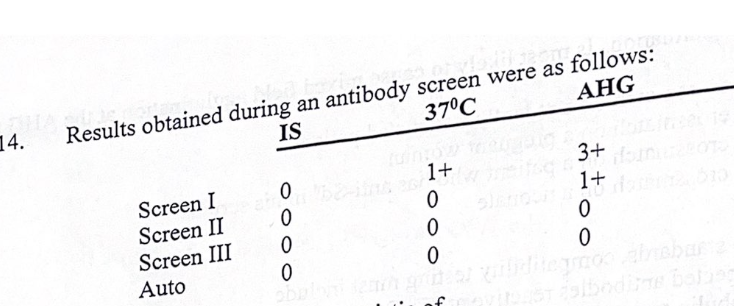
the reaction pattern is characteristic of:
dosage antibodies
results obtained during an antibody screen were:

the reaction pattern is characteristic of:
IgM antibodies
results obtained during an antibody screen were:

the reaction pattern is characteristic of:
warm reactive autoantibodies
Leukocyte reduced red blood cells in- tended for the prevention of febrile non-hemolytic transfusion reaction should contain _
Less than 5 x 10^6 leukocytes
Given that a patient's indirect antiglobu- lin test is negative, which of the following may cuase a positive result in a major crossmatch?
- incorrect ABO typing of the patient
- an alloantibody against an antigen on the donor cells
- prior sensitization of donor RBCs with antibody
All are correct
A minor crossmatch is performed using ___
donor serum and recipient red cells
A blood specimen is received in the blood bank with the request for trans- fusion. The tube has the patient's first and last name and the date and time of collection on the label. What else must be on the label according to AABB stan- dards?
The patient's unique identification num- ber
A patient has several specimens in the blood bank from previous testing. Which of these specimens is suitable if blood is required STAT?
specimen 1 day old.
A patient has a hemoglobin of 7.0 g/dl. The surgeon wants a Hgb of 9.0 g/dl before doing surgery. How many units of blood will the patient need to bring the Hgb to the required level?
2
The major crossmatch involves testing ___
Recipient serum and donor cells
What performing a major crossmatch, all of the following incompatibilites will be detected except
- Group A donor and group O recipient
- Group B donor and group A recipient
- Kell positive donor and a recipient with anti-K
- Rh positive donor and an Rh negative recipient with a negative antibody screen
Rh positive donor and an Rh negative recipient with a negative antibody screen
A 24-year-old female with a diagnosis of MM tests as follows:
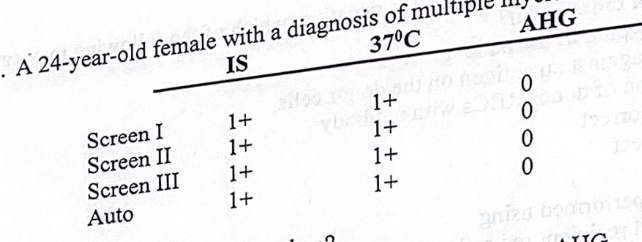
what’s the next step to be taken?
look at agglutination under the microscope
A 24-year-old female with a diagnosis of MM tests as follows:

the most probable cause of the incompatibility is
rouleaux formation
When a recipient has been transfused or pregnant within the past 3 months, how soon before transfusion must a speci- men be collected for crossmatch?
72 hours
According to AABB standards, tests for compatibility shall employ methods that demonstrate ABO compatibility, as well as clinically significant unexpected anti- bodies that are reactive at ___
37c and AHG
The process of removing an antibody from the RBC membrane is ___
elution
At the end of an antiglobulin test, Coombs control cells are added to the negative tests. If agglutination occurs ___
The test is valid
Which of the following will the major crossmatch do?
- Prevent immunization
- Prevent delayed transfusion reactions - Guarantee normal survival of the RBCs -Generally verify donor ABO compatibil- ity
generally verify donor ABO compatibility
Which of the following will be incompati- ble in the major crossmatch?
A pos recipient and AB pos donor
Based upon Kleihauer-Betke test re- sults, which of the following formulas is used to determine the volume of fetoma- ternal hemorrhage in ml of whole blood?
% of fetal cells x 50
The major crossmatch will detect ___
Recipient antibody directed against anti- gens on donor RBCs
Pretransfusion compatibility testing must include ___
antibody screen at AHG
A 22 y/o male is admitted to the ER with a massive hemorrhage from knife wounds to the chest and abdomen. Emergency transfusion is required. What would you issue during emergency release?
O positive red cells
Chills and fever are symptoms of which of the following transfusion reactions?
febrile
The most common type of HDN is due to antibodies of the ___ system
ABO
For an antibody to cause HDN, is must be of which immunoglobulin class?
IgG
The antigen that stimulates the produc- tion of antibody in HDN ___
Is a paternal antigen shared by the fetus but absent from the mother
The most severe cases of HDN are due to antibodies of the ___ system
Rh
What is
- not indicated in ABO HDN
- indicated if the mother has had a previ- ously affected baby
- is not indicated if only IgM antibodies are present
Aminocentesis
Which of the following is true of blood used for exchange transfusion?
- It must always be the same ABO as the mother
- It must always be the same Rh as the mother
- It must be negative for the antigen to which maternal IgM antibody is directed - It must be negative for the antigen to which maternal IgG antibody is directed.
It must be negative for the antigen to which maternal IgG antibody is directed.
If a physician wants to know if there has been a fetomaternal bleed at 28 weeks gestation, which test would you perform?
Kleihauer-Betke stain
A single vial vial of Rh immune globulin should be used to counteract the immu- nizing effect of __
30 ml D positive whole blood
An adopted 3-day-old neonate with a positive DAT needs a transfusion. You would ___
Perform an elution on the baby's red cells and use the eluate to crossmatch blood
When group specific FFP is not avail- able, a group AB individual could alter- natively be given ___
no alternative
A group A individual could receive __
group A red cells
Implementation of a maximum surgical blood order schedule should result in __
an increase in the number of type and screen procedures
Which filter should be effective in pre- venting febrile transfusion reactions?
leukocyte reduction filter
All of the following are appropriate vol- ume expanders except
- dextran
- normal saline
- hydroxyethyl starch - fresh frozen plasma
FFP
In which circumstance would it be feasi- ble to transfuse Rh-positive blood to an Rh-negative individual?
elderly woman
direct coombs test purpose
tests for the presence of patient antigen. This test detects in vivo coated RBCs. The DAT is used to detect AIHA, drug-induced hemolytic anemias, HDN, and HTRs. The procedure for this test is to prepare a 3-5% suspension of patient red blood cells, wash one drop of that solution in the cell washer, add two drops of anti-human globulin (AHG) into the tubes. One tube is spun for 15 seconds. The other tube sits for 5 minutes to allow any potential complement to react in the tube. The cells are then resuspended and read for agglutination. If the cells do not agglutinate, one drop of Coombs control cells is added, and then centrifuged to ensure the cells are washed properly. If the Coombs control cells do not agglutinate, the results of the antibody screen are invalid and the test should be repeated.
indirect coombs test
tests for the presence of patient antibody. This test detects in vitro RBC coating by an antibody. IAT can also be used for compatibility testing and red cell phenotyping. Instead of using cells from the patient, the test uses screening cells (cell I and II). Three drops of patient plasma are placed in the tubes, and then the reagent cells are added next. The cells are mixed for 15 seconds, and then LISS (low ionic salt solution) is added then. The cells are centrifuged for 30 seconds and then the cells are washed three times with 0.9% saline. Then, two drops of anti-IgG are added to each tube, and centrifuged for 15 seconds. Any agglutination is recorded, and all negative results are tested with Coombs control cells. These cells are mixed and centrifuged for 15 seconds before being observed for agglutination. If the cells do not agglutinate, then the screen is invalid.
compare/contrast indirect and direct coombs tests
In both instances the antisera is placed first, and then cells are added afterwards. Indirect Coombs uses LISS and direct Coombs does not. Both use anti-IgG in testing, and both use a cell washer to get rid of excess antibody. Coombs controls are implemented to ensure that the cells are washed efficiently.
2. Why is it important to wash RBCs free of serum before the addition of AHG?
It is important to wash RBCs free of serum before adding AHG because excess antibody not attached to red blood cells should not be present in the sample, and may cause false negative results if not properly washed. This would cause false negative results, and the Coombs control cells would agglutinate.
What antibodies are found in polyspecific AHG serum?
Polyspecific AHG serum contains anti-C3d and anti-IgG.
What is the purpose of Coombs cells in AHG testing?
The Coombs cells in AHG testing are used to check if the red blood cells were washed adequately. If they are washed properly, then the check cells would agglutinate because they can properly bind to the surface
List the properties of complete antibodies.
regularly agglutinate in saline, are not detected by AHG, readily activate complement, is naturally occurring, reacts optimally at room temperature or lower, and does not cross the placenta. One example of complete antibodies is IgM. The IgM is a pentamer with five antigen binding sites and five complement binding sites. Another complete antibody is IgA.
List the properties of incomplete antibodies.
Incomplete antibodies do not regularly agglutinate in saline because they are too small, is revealed by AHG, is not very effective at activating complement, is stimulated by red cells, and is clinically significant. This type of antibody reacts best at 37°C, crosses the placenta, and can cause hemolytic disease of the newborn and is also revealed in hemolytic transfusion reactions.
What is the purpose of an antibody screen? What are reagent cells I, II, and III?
The purpose of an antibody screen, including the DAT or IAT, is to determine if antigen-antibody complexes are formed in the patient blood or serum. If this is the case, then further testing should be done to determine the specific antibody.
Reagent cells I, II, and III contain various patterns of CDE in the blood. These screening cells are O cells phenotyped for the most common antigens of the population and the antibodies that are the most clinically significant. Reagent cell I contains R1wR1, or CDe/CDe with a weak or variant C. Cell I is anti-C. Reagent cell II is R1R1, or CDe/CDe. Cell II is anti-D. Reagent III is R2R2, or cDE/cDE. Cell III is anti-E.
What is a red cell panel and what is it used for?
The red cell panel is used for identifying what type of polyagglutination is happening in patient cells. If the patient's serum reacts with cells I, II, or III, then this indicates that the patient may have other antibodies that could adversely react with blood transfusions. For prenatal patients, the panels can look for clinically significant antibodies that may cause hemolytic disease of the newborn.
Why are different temperatures and mediums used in antibody detection and identification?
Different temperatures and mediums are used to detect antibodies because IgG antibodies react best at 37°C, while IgM and IgA antibodies react best at room temperature or lower. IgM and IgA are also seen to react right away in the immediate spin. Different mediums such as exposure to LISS is used for IgG reactions because it allows the reaction to be enhanced, so that the red cells can properly bind to the IgG.
What is zeta potential? How does the use of albumin and enzymes effect the zeta potential?
Zeta potential explains why red cells suspended in saline repel each other, making it hard to agglutinate with small IgG molecules. The red cells themselves are surrounded by a cloud of net negative ions, or an "ionic cloud." When the negatively charged clouds surrounding the red cells repel each other, the red cells do not spontaneously agglutinate. Specifically, the zeta potential measures the difference in charge between the membrane surface and the outer edge of the ionic cloud. Albumin and enzymes help to decrease the zeta potential and increase the dielectric constant, or decrease the number of ions in the ionic cloud is between two red cells. When the dielectric constant of the cells is high, the electrical conductivity charge successfully dissipates in the medium. Enzymes specifically release sialic acid, the RBC membrane residue. This is how the zeta potential decreases and the dielectric constant increases. When enzymes and albumin are used in a cell solution, this allows for antibody-antigen reaction to become more prevalent.
Explain why LISS is used in antibody detection and identification?
Enhancement reagents like LISS and PEG promote promote antigen-antibody reactions by bringing the antibody- coated cells closer together so that the Coombs reagent can "bridge" a gap between them. It functions by reducing the zeta potential of the RBC, meaning that it decreases the number of ions present in the ionic cloud surrounding red blood cells, decreasing ionic strength and increasing the antigen-antibody agglutination rate.
What are typical hematological and immunological/blood bank findings of a warm autoimmune hemolytic anemia, and why?
37°C autoantibodies.
cells are coated with red cells and complement.
spleen sequesters the IgG antibody and spherocytes tend to be common
shortened cell survival time, and these patients also have a decreased hemoglobin and anemia.
No free hemoglobin is released into circulation.
relate to the hematological disease lymphoma, or other diseases like carcinoma or systemic lupus erythematosus.
positive DAT of anti- complement or anti-IgG. If the reaction is positive for an IgG, the MLS can perform an elution to determine the specificity. The cold acid elution technique is used to determine this result.
Organic solvents or heat elution techniques separate the antibody from the antigen. The eluate test and the antibody identification panel can be used to determine specificity.
high LDH, bili from hemolysis, all screen cells and DAT are +, autocontrol + with some reactivity
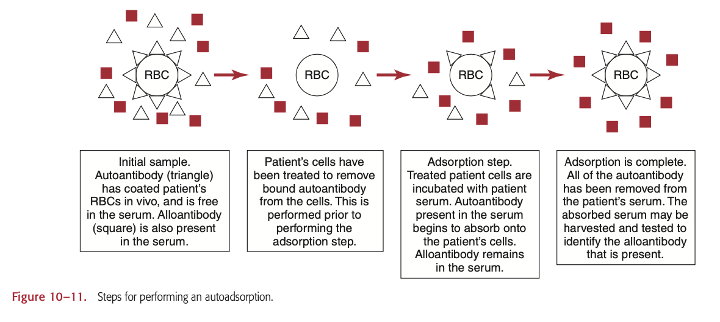
Are there risks involved in performing warm autoadsorptions on recently transfused patients?
Autoadsorption is the simplest method to removing autoantibodies, since it uses patient RBCs.
Some risks involved in performing warm autoadsorptions on recently transfused patients include false reactions due to improper washing of the patient RBCs. This method works best when the patient has not been recently transfused, within 3 months because the cell antigens that react with patient antibodies must match for the reaction to work well.
According to AABB standards, how long may a sample safely be used for compatibility testing?
According to AABB standards, a sample can safely be used for compatibility testing for up to 72 hours (3 days) after collection.
What rule must be followed when transfusing group O blood to another blood group such as an A or В?
Group O blood can be transfused to other blood groups like group A or B, but a type and cross match should be tested to make sure that the patient does not have a negative reaction. For example, if the type O donor blood has other subgroups of A or B that are less common, this could also cause a negative reaction for the patient. The O blood should be crossmatched with anti-AB.
What is rouleaux formation? How is it differentiated from true agglutination?
Rouleaux formation occurs when there is excess proteins in the blood, from conditions like multiple myeloma. It can also be caused by plasma expanders and Wharton's jelly. The blood appear like a stack of coins, and can be mistaken as agglutination. It can be differentiated from true agglutination because agglutination is shaped more like a clump, while rouleaux is even and orderly. When the rouleaux is washed multiple times with saline, then rouleaux is dispersed.
What would agglutination or hemolysis in the immediate spin phase of crossmatching suggest?
complete antibodies like IgM are present in the patient's serum
ABO incompatibility (only if the patient doesn’t have any other clinically significant antibodies)
OR cold agglutinin
The neonatal intensive care unit has requested FFP STAT for a neonate. No blood specimen is available for typing. What type of plasma would you thaw? Consider ABO /Rh type as well as special considerations for neonates.
Neonatal intensive care unit thaws AB irradiated plasma since the AB plasma does not contain anti-A or anti-B, so there is a minimal chance for a negative reaction. Neonates need this type of plasma since their immune systems have not developed yet. The neonate plasma is in a much smaller container than regular plasma units.
A pre-operative type and screen was ordered on a 43-year-old male. The patient tested as O positive with a negative antibody screen. Previous records show a history of anti-Jka 5 years ago. How would you handle this case?
When the patient has a history of anti-Jka 5 years ago, the history of anti-Jka should still be considered cautiously before transfusion. The patient and donor units should be typed for the Jka antigen, and then the donor Jka negative units should be crossmatched with the patient's serum to ensure no reaction would occur. In order to confirm the presence of this antigen, using enzymes could help confirm its in the patient. Even if the anti-Jka was no longer present, the patient would still need to get anti-Jka blood.
What is an immediate spin crossmatch? Who is eligible for this procedure? Why is it considered safe?
An immediate spin crossmatch is a room temperature immediate spin that detects ABO discrepancies. The IS crossmatch is useful for recipients with no clinically significant antibodies or any history of antibodies. This crossmatch is used when the patient does not need blood for certain. It is considered safe because as long as the antibody screen remains negative for clinically significant antibodies, then the patient has a low chance of reacting with the donor blood.
Select group specific and group compatible red cells for the following phenotypes:
A neg
AB pos
O pos
A pos
B pos
AB neg
A neg: A = and O =
AB pos: All blood types; A+ and =, B+ and =, AB+ and =, and O+ and = O pos: O + and =
A pos: A+ and =, O+ and =
B pos: B+, O+
AB neg: A=, B=, AB=, and O=
A 40-year-old female is scheduled for surgery. Her antibody screen is negative at all phases. Of 6 units crossmatched, 5 were compatible and one was 1+ incompatible at the antiglobulin phase. List three possible causes for this incompatibility.
This incompatibility could be caused by antibodies attaching to the red blood cell surface. The antibodies attached to the red blood cell surface could be caused by a low incidence antibody that was not detected by the antibody screen, an antibody with a dosage effect may have been present and revealed itself when there was a higher dosage in that one unit, and a technical error or reagent problem could have caused a false positive result. The antibody detection reagent could have also been faulty.
According to AABB standards, donor units that test positive for the weak D antigen must be labeled as _____
Rh positive.
Determine if RhiG is indicated for the following maternal/ cord blood types:
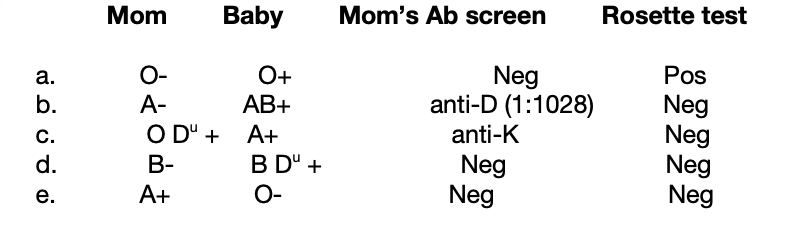
a. yes
b. no: patient already has anti-D
c. yes: patient could still make partial D
d. yes
e. no
An antibody screen performed on an obstetrical patient indicated the presence of a weakly reactive anti-D with a titer of 1:4. After delivery, RhIG was requested for the patient. Mother and baby type as: Mom -A neg, Baby - A pos. Is RhIG indicated? What could be the cause of the weak anti-D?
Rhogam is indicated because the mother is Rh negative and the baby is Rh positive, and the mother already has a weakly reactive anti-D with a titer of 1:4. The weak anti-D could be caused by the mother having a previous pregnancy with an Rh positive baby.
Blood for exchange transfusion was requested for a baby with a bilirubin of 18.0 mg/dl. The mom is O negative with anti-D (1:2048). The baby is A positive with a 4+ DAT due to anti-D. Describe the product selection and preparation.
AND irradiated
Exchange transfusion replaces most of the baby's blood with donor blood, and to determine the correct blood, the donor blood should be crossmatched with the mother. If this is not possible, the cord blood should crossmatch with the donor blood. If the cord blood is not available, then the baby's serum should be crossmatched with the donor blood. The type O blood must be fresh, CMV negative, and hemoglobin S negative. The blood for the baby should be coupled with an appropriate Rh resuspended in AB plasma. The donor RBCs should meet a certain Hct 50-65% once the plasma is added, to be verified by the Hematology part of the lab.
Group A and B babies of _____ often have ABO HDN.
group O mothers
Can ABO hemolytic disease of the newborn be easily predicted on a basis of maternal antibody studies
antepartum?
ABO HDN can not be easily predicted before birth because ABO HDN is not as strong of a reaction as Rh HDN. Antibody screening does not usually detect ABO reactions unless the titer is high enough. But, this type of reaction does tend to occur when the mother's blood is type O while the baby blood type is A1 or B. Group O people tend to have higher titers of anti-A and anti-B. But, ABH antigens are not fully developed at birth, so the reaction and agglutination may be weaker.
If exchange transfusion is required for a B negative fetus suffering from ABO HDN and the mother is O positive, what type blood would you recommend for the transfusion?
The baby should receive Rh= blood O negative blood, which is CMV negative, and hemoglobin S negative. The blood itself should have a high hematocrit and should be resuspended in AB plasma AND IRRADIATED
Which of these antibodies have been implicated in HDN: anti-A, -B, -H, -C, -Fya, -I, -K, - Lea, -e, -Jkb, -N?
Anti-A, anti-B, anti-e, anti-K, anti-Fya, anti-N, and anti-Jkb.
How long may blood be used for transfusion if a unit of whole blood is opened to remove plasma?
Up to 4 hours because the blood is no longer in a sterile environment.
How soon after delivery should RhIG be given to be effective?
RhIG should be given during the 28th week of pregnancy antepartum or antenatally, and also should be given within 72 hours of delivery of an Rh positive infant.
List the indications for use of fresh frozen plasma.
Fresh frozen plasma should be used for multiple factor deficiencies, dilutional/consumptive coagulopathies, surgery/trauma, liver disease, Warfarin treatment, factor deficiencies (II, V, X, XI), and TTP.
When should blood be irradiated before transfusing? What does irradiation do to the unit?
Blood should be irradiated before transfusing a baby, all directed donations, any immunocompromised or immune susceptible patient, and anyone else at risk for GVHD. The 25 Gy gamma irradiation destroys T lymphocytes, preventing Graft vs Host disease (GVHD).
By how much should a unit of random donor platelets raise the platelet count of a non-bleeding adult of average size?
A unit of random donor platelets should raise the platelet count of a non-bleeding adult by at least 5.5x10^10 platelets each unit.
small unit: random donor
platelet is 5-10,000
pheresis is 20-60000 increase
What is the micron size of a standard blood filter?
The micron size of a standard blood filter is 170-260 microns.
Do cryoprecipitate and platelets need to be filtered during transfusions?
Cryoprecipitate and platelets do need to be filtered during transfusions. The platelets have a leukoreduction filter and the cryoprecipitate has a standard blood filter.
What is the preferred and maximum recommended infusion time for a unit of red cells?
The preferred and maximum recommended infusion time for a unit of red cells is 4 hours. If the patient cannot handle that much blood in one sitting, the blood can be divided into aliquots so that it can be administered while also being properly stored.
Two units of blood are returned in a cooler from the operating room. Upon checking the cooler temperature, you found it to be 12° C. What would you do with these units?
RBCs should remain at 1-6°C. Its shipping temperature should be 1-10°C. If it is above this temperature, the units of blood should be discarded.
List 5 findings that would cause you to reject/discard a unit of packed RBCs?
Bacterial Growth/contamination
Expired unit
Incorrect temperature
Agglutination/visible clots
hemolysis
Puncture or hole in packaging
Discoloration
According to AABB standards, leukocyte reduced red cells should contain no more than 0.01% of leukocytes.
Or 5x10^6 residual leukocytes.
What is the significance of 2,3-DPG in neonatal exchange and massive transfusion?
2,3-DPG in neonatal exchange and for massive transfusion is important because it regulates and maintains hemoglobin function. In a hypoxic state, hemoglobin has a lower affinity to oxygen to allow a quicker delivery to the tissues. During transfusions, the stored RBCs have lower 2,3-DPG levels because the blood is in a hypoxic state. The 2,3-DPG is then reformed when transfused into the patient restoring oxygen delivery for the patient. (Pg. 8 in textbook)
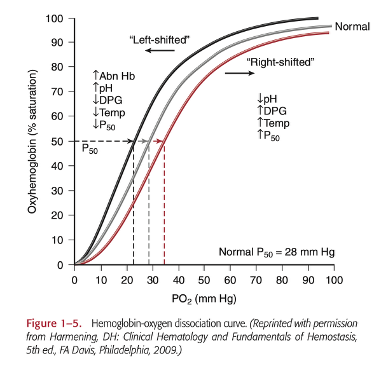
Describe 2 types of transfusion reactions and the clinical symptoms that accompany each. (You should know all the Tx reactions and clinical symptoms).
Cutaneous allergic reactions may be caused by soluble constituents in the donor plasma. Some symptoms include hives, rash, itching, and no fever. For treatment, the patient will respond to antihistamines.
IgA-deficient patients who have formed anti-IgA antibodies may experience an anaphylactic reaction to the blood transfusion. This type of reaction only needs a small amount of blood in order to have a strong reaction. Some of the symptoms include GI upset, a sudden drop in blood pressure within minutes, shock and loss of consciousness, and potential death if not treated quickly. Any following transfusions need to be thoroughly washed from IgA deficient donors.
What tests need to be done in the investigation of a transfusion reaction?
In order to investigate a transfusion reaction, all the blood, fluids, and tubing are sent back to the blood bank. A purple top is drawn from the patient and a random urine is collected and also sent to the blood bank. A clerical check should be performed on the papers, computer, and the specimen. A DAT should also be performed to see if there is an antigen-antibody reaction. Some tests that may need to be redone include the ABORh, antibody screen, and compatibility tests. Other tests may also need to be ordered by the blood bank director.
Bacteriologic studies, coagulation studies, bilirubin, haptoglobin, plasma hemoglobin, and evaluating the urine for hemolysis may also need to be observed.
What are three diseases transmitted by transfusion of blood and blood products?
Three diseases transmitted by transfusion of blood and blood products include Syphilis, Lyme Disease, and Malaria.
Which blood group system causes the most severe transfusion reactions?
The ABO system causes the most severe transfusion reactions.