Anti-Depressants
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Types + Pathophysiology
1. Unipolar:
Exogenous / Reactive Depression
Endogenous/Major Depression
2. Bipolar : Manic – depressive illness
Monoamine Hypothesis
Depression caused by decreased levels of monoamines, serotonin, norepinephrine, dopamine at neuronal synapses.
mania caused by overproduction of NT
Supporting evidences
Use of reserpine (Depletes monoamine stores) → depression
Administration of synthesis inhibitor of NE → depression
All antidepressant drugs enhances the synaptic availability of serotonin and NE
Limitations:
antidepressant effects not seen until 2-4 wks of treatment
overly simplistic
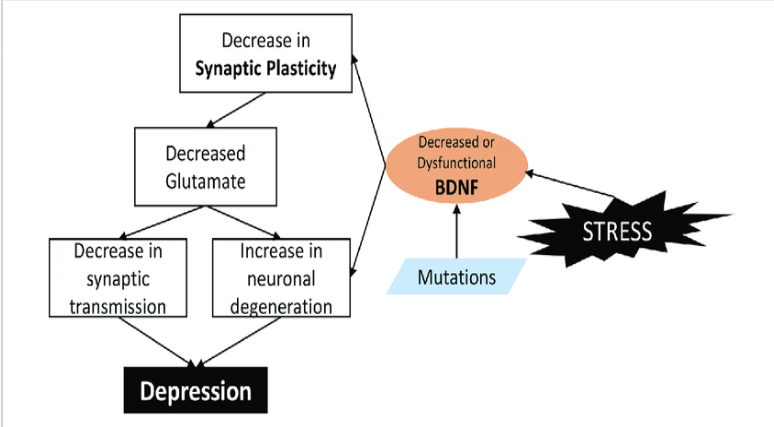
Neurotropic Hypothesis
neurotropic growth factors like brain derived neurotropic factors (BDNF) critical for neurogenesis and plasticity → enhance neurogenic transmission.
tyrosine kinase receptor B → activates enzymes → transcription of certain genes responsible for enhanced synaptic plasticity and connectivity.
Pain/stress/depression → dec BDNF → atrophic changes in hippocampus & cortex.
Evidences in favour of neurotrophic hypothesis
Direct infusion of BDNF in hippocampus → enhanced neurogenesis + antidepressant action
Electroconvulsive therapy increases BDNF
Antidepressants increases BDNF
Neuroendocrine factors
HPA axis abnormalities
cortisol & GC → depression.
Thyroid dysregulation
Hypothyroidism → depression
thyroxine is given along with antidepressant to augment its effect
Deficiency of sex hormones –
Estrogen deficiency (fem) and testosterone deficiency (males) → depression
SSRIs PK
Orally active
Well absorbed after oral administration
PPB – 80-90%
Fluoxetine → Norfluoxetine → t1/2 7 to 9 days
once weekly dose → take 2-12 weeks to produce significant improvements in mood
Fluoxetine, paroxetine → CYP2D6 inhibitor
Fluvoxamine → CYP3A4 inhibitor
SSRIs MOA and Uses
Serotonin transporter (SERT) embedded in axon terminal and cell body of serotonergic neurons → responsible for reuptake of serotonin from synaptic cleft
EC serotonin binds to SERT → conformational change → Na+ and Cl- moves into the cell → serotonin is released inside the cell.
SSRIs binds to SERT and selectively inhibit it from a site other than serotonin site
this serotonin is then used for transcription of genes for neurotropic factors → AD effect
AT therapeutic doses 80 % activity of transporter is inhibited.
Negligible affinity for NET, β , histamine, muscarinic and other receptors.
Clinical Uses of SSRI
Depression
Anxiety/panic disorder - generalized anxiety disorder + social anxiety disorder
Post traumatic stress disorder
Obsessive compulsive disorders
Bulimia nervosa → fluoxetine
Pre-menstrual dysphoric disorder
SSRIs A/E
GIT
nausea
gastrointestinal upset
diarrhea
GI s/s improves after first wk of treatment
take with food
Sexual dysfunction
Decreased sexual functions
Decreased libido, both males and females
delayed ejaculation
anorgasmia
assess at follow up
CNS:
Headache,
insomnia → paroxetine, fluvoxamine → sedating (useful in pts who have difficulty sleeping)
somnolence → fluoxetine, sertraline
Suicidal tendency
patients have increased suicidal tendency
C/I below 25 years of age
fluoxetine, sertraline, fluvoxamine approved for children for OCD
fluoxetine, escitalopram for childhood depression
Serotonin syndrome with MOAI
CVS
citalopram → QT prolongation → arrhythmias
Discontinuation syndrome:
after abrupt withdrawal
chronic SSRI use → inc synaptic serotonin lvls → brain adapts by downregulating serotonin receptors (5HT1A and 5HT2A) → after abrupt withdrawal → drop in synaptic serotonin lvls → adapted brain now receives less serotonin stimulation → neurochemical imbalance → symptoms
caused by agents with short half lives and inactive metabolites
fluoxetine has lowest risk due to its longer half life and active metabolite
headache, malaise, flu-like symptoms, agitation, irritability, nervousness, changes in sleep
Weight gain : Paroxetine
Dizziness & Paraesthesia
reduces seizure threshold → inc seizures
SSRIs DDI - SEROTONIN SYNDROME
Pharmacodynamic – MAOIs
MAOI inhibit monoamine enzyme (responsible for degradation of NE & serotonin) → increased serotonin occurs at neuronal endings → toxic levels
Pharmacodynamic interaction - Serotonin syndrome
Combination of SSRIS with MAOI may result in serotonin syndrome
Overstimulation of 5-HT receptors in medulla
S/S include: SHIVERS → cognitive, autonomic, somatic effects
shivering
hyperreflexia + myoclonus + tremors
inc temperature (>41C)
vital signs instability → hypertension and tachycardia
encephalopathy (cognitive effect → delirium, coma)
restlessness
sweating
To avoid this → gap of 2 weeks given between administration of SSRI and MAO inhibitors.
SNRIs
Pharmacokinetics
Well absorbed after oral administration.
Desvenlafaxine
(active demethylated metabolite of venlafaxine)
inhibitor of serotonin reuptake and at medium/higher doses → inhibits NE
CYP 2D6 inhibitor
lowest PPB (27-30%)
T1/2 →11hrs
OD dosing
Duloxetine (inhibits at all doses)
avoid in pts with liver dysfunction
GI side effects common
t1/2 →12hrs
extensive oxidative metabolism via CYP2D6 & CYP1A2
Mechanism of Action
inhibit serotonin and norepinephrine transporters.
Both serotonin and norepinephrine levels increase → inc transcription of neurotropic growth factors
depression is accompanied by chronic pain such as backache and muscle aches for which SSRIs are ineffective → this pain is modulated by serotonin and NE pathways → SNRIs effective
They lack potent antihistamine, alpha blocking & anticholinergic effects
Therapeutic uses
Major depression
Panic disorders
Diabetic neuropathies & fibromyalgia
Stress urinary incontinence
Vasomotor symptoms of menopause
A/E
Incre. BP
Incre HR
CNS activation: anxiety, agitation & insomnia
Cardiac toxicity with venlafaxine
Hepatic toxicity with Duloxitine
Discontinuation syndrome
Dizziness, paresthesia
TCA
Pharmacokinetics
well absorbed after oral administration
Long t1/2---OD dosing
Metabolized by CYP2D6 (DDI)
Undergo extensive metabolism Demethylation / hydroxylation / conjugation
Mechanism of Action
bind and Inhibit SERT & NET on presynaptic neuron→ inc lvls of serotonin and NE in cleft
SERT - clomipramine / imipramine
NET - desipramine / nortriptyline
alpha blocking (Orthostatic hypotension), anticholinergic & antihistamine (doxepin → pruritis) effects
Affinity of TCA for receptors
Moderate affinity for alpha 1 and alpha 2
Alpha 1 blocking property
Alpha 2 agonistic property
Therapeutic Uses
Endogenous Depression (Refractory to SSRIs)
improves mood in 50-70% individuals
onset of mood elevation is slow (2wks+)
adjust dose according to response
tapering of agents recommended to avoid discontinuation syndrome and cholinergic effects
Panic disorder.
Enuresis in children - bed wetting (imipramine used as an alt to desmopressin)
Neuropathic and other pain conditions (amitriptyline)
Generalized Anxiety Disorder
Obsessive Compulsive Disorder
Attention deficit hyperkinetic Disorder.
Neuralgias
Migraine (amitriptyline)
Smoking cessation (nortryptalline)
insomnia - low doses of TCAs including doxepin
TCA A/E
NE release → hypertension, inc BP (venlafaxine → cardiotoxic)
Anticholinergic effects (amitriptyline, imipramine)
Dry mouth, constipation, urinary retention, blurred vision & confusion
aggravation of angle-closure glaucoma
α blocking property
Orthostatic hypotension & reflex tachycardia
arrythmogenic + quinidine-like property
antiarrhythmic effect but at higher doses → arrhythmias
H1 antagonism
Sedation & wt gain
Discontinuation syndrome
Flu like syndrome
Cholinergic rebound
Overdose/ toxicity with TCA
Vent Tachycardia, fibrillation & arrythmias
BP changes
Anticholinergic effects
Seizures
Management
Airway support, cardiac monitoring, gastric lavage
Sodium bicarbonate to uncouple the TCA from sodium channels
CI
use with caution in pts with bipolar disorder because AD may cause a switch to manic behavior
depressed pts who are suicidal should be given very limited quantities and monitored
exacerbates BPH, epilepsy, arrhythmias
Dis/Advantages of SSRI over TCA
ADVTANTAGES
Easily available
greater safety margin/safe in overdosage
inexpensive
Less frequent anticholinergic & CV A/E
Free of sedation
Also useful in eating disorders
DISADVANTAGES
Frequent nausea at the start of treatment
Sexual dysfunction
Risk of serotonin syndrome
Incre. Potential for D/D interactions
Icreased suicidal tendency
5HT2 Antagonists
Pharmacokinetics
Well absorbed after oral administration.
Extensive PPB
Extensively metabolized in liver
Both drugs are converted into active metabolites, having potent antidepressant activity.
Trazodone is converted to m-cpp (m-chloro phenyl piperazine)
Nefazodone is converted into hydroxy nefazodone and m-cpp
Nefazodone potent CYP3A4 inhibitor
Trazodone cyp 3 A4 substrate
Mechanism of action
block 5HT2A receptors → antianxiety, anti-psychotic and antidepressant effects
Nefazodone: Weak inhibitor of SERT & NET → raising the amine levels
Trazodone: weak inhibitor of SERT with little effect on NET
alpha blocking & antihistamine effects
Therapeutic uses of 5HT2 antagonists
Depression
Anxiety disorder
Insomnia (trazodone)
Adverse effects
GIT upset
Hepatotoxicity with Nefazodone
Sedation → Trazodone → antihistamine effect
Orthostatic hypotension alpha blocking effect
Tetracyclic and Unicyclic AD
Pharmacokinetics
Well absorbed after oral administration.
Extensive PPB.
Extensively metabolized in liver
Bupropion
has three active metabolites including 7-hydroxybupropion
biphasic elimination
1st phase: 1 hour; 2nd phase: 14 hours
inhibits reuptake of NE and dopamine by inhibiting NET and DAT
Stimulates presynaptic release of catecholamines
metabolized by CYP2B6
avoid in seizure/bulimia risk
Mirtazapine
Blocks alpha 2 presynaptic receptors, H1 receptors
Enhances the release of NE & serotonin (serotonergic and adrenergic)
Antagonist of inhibitory receptors 5-HT2A, 5-HT2C and 5-HT3 receptors--- incre. availability of serotonin
no MOA reuptake inhibition
Amoxapine → hydroxy amoxapine → D2 receptor blocking activity → antipsychotic effects
also block NET, SERT and have some anticholinergic activity → caution when administered together
Clinical uses
Depression unresponsive to other therapies
Bupropion – obesity, smoking cessation & ADHD
decreases cravings and attenuating withdrawal relating to nicotine
Adverse effects
Sedation → mirtazapine, bupropion → antihistaminic effect
Parkinsonism → Amoxapine → D2 antagonist
Maprotiline → TCA like effects
Bupropion → causes stimulation leading to agitation, insomnia, anorexia.
Hydroxy bupropion is 2D6 inhibitor
CI in pts taking MAOI
MAOI
Pharmacokinetics
orally active
Extensive hepatic first pass metabolism
Alternative routes like transdermal and S/L preparation of selegiline → by pass gut & liver (no FPE) → incre B.A.→ dec food interactions
selegiline and tranylcypromine → amphetamine-like stimulant effect → agitation/insomnia
Mechanism of action:
“safety valve” to oxidatively deaminate and inactivate excess NTs that leak out of synaptic vesicles when neuron is at rest
MAOI form stable complexes with the enzyme → irreversible activation → inc stores of NE, serotonin, dopamine in neuron + diffusion of excess NT in synaptic space
inhibits MAO-A and MAO-B
MAO-A
present in noradrenergic, dopaminergic neurons
degrades NE, epinephrine, serotonin, dopamine and tryptamine.
found in gut, liver, placenta and brain.
MAO-B
present in serotonergic & histaminergic neurons
degrades monoamines, phenylethyl amine, tyramine, dopamine and tryptamine.
present mainly in brain, platelets and liver.
Clinical uses
used for pts who are unresponsive or intolerant to other AD (not preferred due to their DD and drug-food interactions)
Anxiety disorder
Selegiline –Parkinson’s disorder
Adverse effects
Orthostatic hypotension
Weight gain
Sedation (Phenelzine)
Amphetamine like effects → CNS stimulation, insomnia, irritability, restlessness
Dec sexual functions
Discontinuation syndrome
Psychosis, excitement, confusion, delirium
Drug interactions
Cheese reaction/tyramine pressor response
normally tyramine is taken from food and extensively metabolized by MAO in gut → less BA.
Food containing tyramine given to a patient on MAOI.
MAO is blocked → tyramine is not degraded → pass from gut to circulation + sympathetic nerve endings
Acting as indirectly acting sympathomimetics → displace norepinephrine from nerve endings → incre. availability of NE → malignant hypertension, stroke, cerebral hemorrhage & MI (hypertensive crisis), occipital headache, arrhythmias
Foods like cheese, beer/red wine, sausages, liver, smoked fish are avoided in pts taking MAOI.
SSRIs shoudl not be co-administered with MAOI → serotonin syndrome
washout period of 2wks before other type administered (except fluoexetine which should be discontinued 6wks before MAOI adm)
Overdose of MAO inhibitors
Autonomic instability
Psychotic symptoms
Fever
Confusion
Delirium
Seizures
Management
Cardiac monitoring, vital
support & lavage
NMDA receptor Antagonists
Ketamine & esketamine NMDA receptor blocker
These drugs have multiple other actions that can contribute to efficacy in depression
These include interactions with opioids receptors, monoamine receptors, cholinergic receptors & ca channels.
Ketamine enhances activity of descending setonergic pathways that may be imp for antidepressant property
GABAA receptor Modulators
GABAA receptors are ligand gated chloride conducting ion channels that causes inhibition of neural networks including those associated with limbic overactivity in maj depression
Brexanolone resets the dysregulated brain function in depressive episodes through modulation of GABAA receptors
Electroconvulsive Therapy
For severe suicidal depression
Electrodes r placed on head & give convulsions--- incre NT levels