The History of the Atom - Yr 11 Chem ATAR
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
When did John Dalton publish his model?
. Published his atomic model in 1808, detailing his theory in a book titled “A new system of chemical Philosophy”
What 3 pieces of evidence were provided to support John Dalton’s model?
. Law of Partial Pressures (Dalton’s Law): He discovered that the total pressure exerted by a mixture of gases is equal to the sum of the pressures of each individual gas. This suggested that gases were made of separate particles rather than being continuous substances.
. Gas Absorption in Water: Dalton observed that different gases dissolve in water at different rates, which he attributed to differences in atomic weights.
. Law of Multiple Proportions: Dalton found that when two elements form multiple compounds, the ratio of the masses of one element that combine with a fixed mass of the other is always a small whole number. For example, carbon and oxygen can form both carbon monoxide (CO) and carbon dioxide (CO₂), with a simple ratio of 1:2 for the oxygen atoms
What was John Dalton’s atomic model determined from his experiment/research?
. Called the Billiard Ball/Solid Sphere model as he envisioned atoms as solid spheres like billiard balls
6 main propositions of John Dalton’s theory
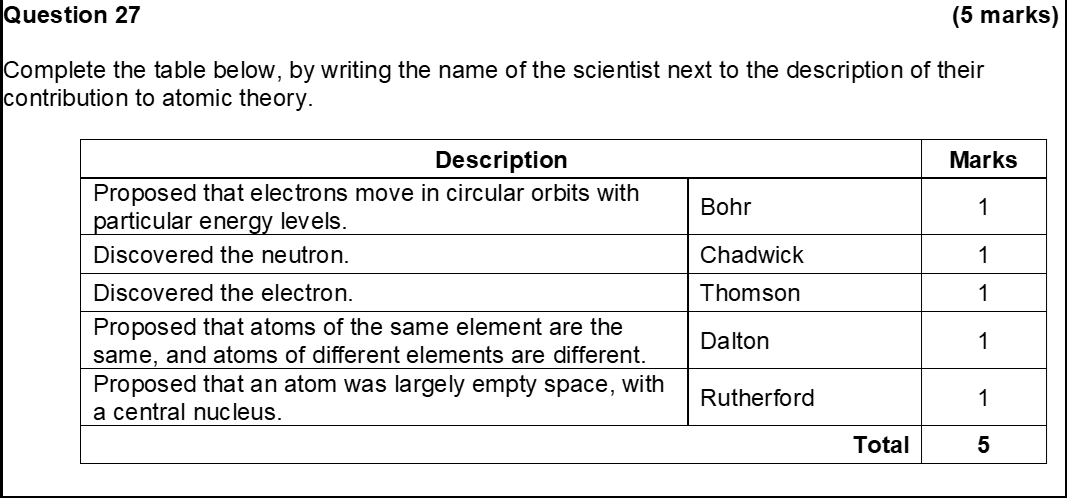
1. All matter (elements) is comprised of tiny, definite particles called atoms
2. Atoms are indivisible and indestructible (meaning atoms aren’t created nor destroyed or changed into different types during a chemical reaction) à incorrect
3. A chemical reaction involves only separation, combination or rearrangement of atoms
4. All atoms of a given element are identical having the same size, mass and chemical properties
5. Atoms of different elements have a different size, mass and chemical properties
6. Compounds are formed when atoms of more than one element combine in a fixed, whole number, specific ratio
John Dalton atomic theory diagram
How did John Dalton’s model improve the understanding of the atom?
. his model significantly improved our understanding of atomic structure by introducing key principles (stated above) that explained chemical reactions and matter’s composition
. He established the concept of atoms (1), providing a scientific explanation for the nature of elements
. He defined elements and compounds (4,5,6), supporting the law of definite proportions
. Explained chemical reactions (2,3), reinforcing the law of conservation of mass
What previous model did Dalton’s improve on?
. Before Dalton, concept of atom was essentially just theoretical, based largely on the ideas proposed by ancient Greek philosopher Democritus, in which he suggested that all matter in the universe was made up of tiny, indivisible, solid objects called atomos
. Thus, Dalton’s model was the first to provide a scientific framework for the understanding of atoms, transforming the atom from a philosophical concept into a credible scientific idea
What model superseded Dalton’s
. Thomson’s plum pudding model superseded this model, which includes the inclusion of a newly discovered subatomic particle called an electron.
When did Thomson publish their work?
Published his atomic model in 1904 (discovered electron in 1897 though)
What experiments did Thomson conduct as part of his research?
. Used the cathode ray tube experiment to discover the electron
. A cathode ray is a vacuumed glass bottle that has a positively charged cathode at one end and a negatively charged anode at the other end. These 2 ends are connected by an electric charge and cause an invisible beam to be produced. Thomson could see this beam since he used zinc sulfate on the tube
. Thomson noticed that when he applied electric plates and a magnetic field the beam bent towards the positive charge and away from the negative charge suggesting that the rays were made of a stream of negatively charged particles with mass
. Was also able to determine the mass of the particles based on their charge to mass ratio that he determined from their deflection from a magnetic field. Using this he was able to calculate that they were 1/2000th of the mass of the atom. Since atoms made up everything and this new particle was smaller than an atom, the electron must’ve therefore been found inside the atom
. Also noticed that no matter what metal the anode and cathode was made of, they still produced the ray meaning that these electrons made up the atoms of all elements
What was Thomson’s atomic model determined from the experiment?
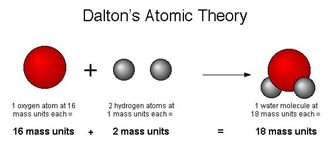
. Plum Pudding model: proposed that an atom is a sphere (sea) of positive charge with negatively charged electrons embedded within it, similar to raisins in a plum pudding, where the positive charge and mass is spread uniformly/evenly throughout the atom and the electrons are free to move within this positive sphere. Most of the mass of the atom was concentrated in the sphere of positive charge
. Knew the atom must be made of a sphere of positive charge because the atom had an overall neutral charge so there must’ve been a positive charge to balance out the negative charge of the electrons
Diagram of Thomson model
How did Thomson’s model improve our understanding of the structure of the atom?
. improved the atomic model by discovering the electron, which was the first subatomic particle of the atom to be discovered
What previous model did Thomson’s improve on?
. Improved on the Billard Ball model made by Dalton, by adding subatomic particles, known as electrons
. Thomson’s model proved that the atom isn’t indivisible since it includes smaller particles of electrons
. Thomson also showed that parts of the atom were positively charged, while different parts were negatively charged, going against the beliefs of Dalton who thought the entirety of the atom was neutral
What model superseded Thomson’s model?
Ernest Rutherford’s nuclear model
When did Rutherford publish his model?
. Published his nuclear atomic model in 1911
What experiment did Rutherford conduct as part of his research and what evidence did this provide to support his model?
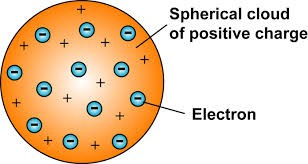
. Conducted the gold foil experiment (1909):
. A beam of 2+ positively charged alpha particles emitted by a sample of radium, was fired at a thin (since very malleable) sheet of gold foil. The gold foil was surrounded by a screen coated with Zinc Sulfide that detected where the alpha particles collided (screen would illuminate on every collision). The expectation was for all the alpha particles to pass straight through the gold foil, since according to Thomson’s model, the spread out (low density) positive charge of the atom wouldn’t be strong enough to alter the path of the alpha particles.
Evidence:
. 99.9% of the time, the alpha particles went through undeflected (evidence that atoms are mainly composed of empty space), 0.07% of the time, the alpha particles would deflect more than expected as they would travel close to the positive nucleus of the gold atoms and slightly repel away from it as both positive charges, and 0.03% of the time, the alpha particles would deflect backwards as they would make contact with the gold nucleus.
Rutherford Experiment diagram
What was the atomic model determined from Rutherford’s research?
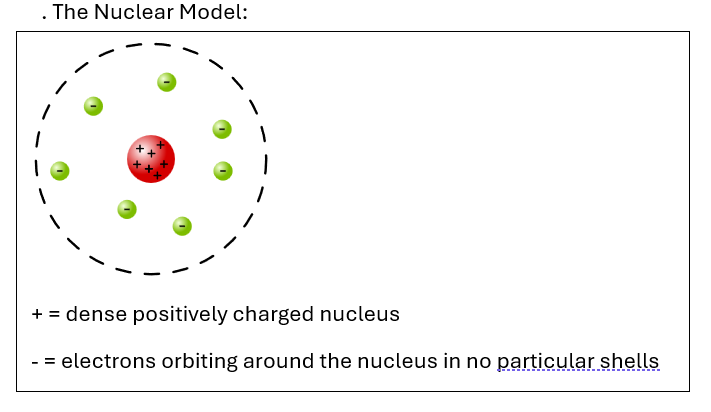
How did Rutherford’s model improve the understanding of the structure of the atom?
. Discovered atoms have a small, dense, positively charged nucleus (containing protons), creating the nuclear model of the atom. The nucleus is tiny but contains over 99% of the mass of the atom
. Electrons orbit around the nucleus (however their arrangement wasn’t yet well defined)
. Most of the atom is empty space, with the electrons making up most of the volume (nucleus takes up very small volume)
What model did Rutherford improve on?
. improved on Thomson’s plum pudding model, changing the model completely by realising the positive charge of the atom was concentrated in a tiny dense nucleus rather than evenly distributed across the atom
. Also discovered that the electrons orbited the positive nucleus rather than being embedded inside of it
What model superseded Rutherford’s model?
. Nuclear model was superseded by Niel Bohr’s Planetary model of the atom
When did Bohr publish his model of the atom?
. Published the first of 3 papers on his atomic model in July 1913
What experiments did Bohr conduct as part of his research?
. Didn’t do many of his own experiments, instead mainly interpreted the results of other people’s existing experimental data, while he also did theoretical calculations
. One piece of evidence he used to prove his model: The Franck-Hertz experiment - demonstrating that electrons can only absorb energy in discrete amounts (quantised energy levels), which directly aligns with Bohr's theory of electrons occupying specific energy levels within an atom
. Also used the emission spectrum of hydrogen: a discharge tube contained hydrogen gas, and as a high voltage was added to tube, the hydrogen atoms became excited and then Bohr observed the different colours emitted by the H through a glass prism
. Thus, when hydrogen is provided with energy, it will release a few very specific wavelengths of light. Bohr’s theory was able to explain this by stating (when provided with energy) that the electrons of hydrogen would gain energy and jump up from one energy shell (their low energy ground state shell) to the next higher energy level shell (excited state), before jumping back down due to the atom being unstable. When the electrons return from their excited state back to their ground state, they release the excess energy as a photon, with energy emitted equal to the energy difference between the shells that they have just travelled between.
What was Bohr’s atomic model determined from his research?
The Planetary model
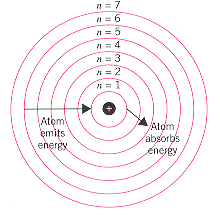
How did Bohr’s model improve the understanding of the structure of the atom?
. Improved our understanding of the atom by discovering that electrons can only exist in certain energy levels/shells around the nucleus.
. This helped to explain a major problem with Rutherford’s nuclear model which was that eventually the electrons would run out of energy and collapse into the nucleus.
. Also discovered that electrons could jump from one shell to another when they gain energy, going from their ground state to their excited state, where excess energy is absorbed on the way up and emitted as photons on the way down, accounting for the emission spectra
What model did Bohr’s Improve on?
. Improved on Rutherford’s nuclear model
. Added to the model: While Rutherford theorised correctly that electrons orbited around the nucleus, Bohr added to this by demonstrating they could only orbit around the nucleus in specific radii, with each of these radii corresponding to the energy of the electrons (introducing quantised electron orbits, explaining atomic stability), with electrons able to change levels from a gain (absorbing) or loss (emitting) of energy (explaining spectral lines)
. Was also able to account for the reasoning why the electrons didn’t collapse into the nucleus when orbiting, as while revolving in their discrete orbits, the electrons don’t radiate energy
What model superseded Bohr’s model?
. Planetary model was improved over time with the discovery of the neutron and the proton, and superseded by the quantum mechanical model by Erwin Schrodinger and Wolfgang Pauli
When did Chadwick publish his model?
. 1932, in his paper titled “The Existence of a Neutron”
What experiments did Chadwick conduct as part of his research?
. Copied experiment from a previously done experiment by irene curie
. The experiment: He directed alpha particles at a beryllium target. The reaction produced a neutral radiation, initially suspected to be gamma rays. He then placed paraffin wax (rich in hydrogen) in the path of the radiation. The unknown radiation ejected protons from the wax, which could be detected. By measuring the speed and energy of the ejected protons, Chadwick calculated that the radiation had a similar mass to a proton but carried no charge, ruling out gamma rays, as they would not have enough momentum to displace protons so effectively. Thus, the mass and charge calculations confirmed the existence of a neutral particle with a mass nearly equal to that of a proton. This discovery explained the missing mass in atomic nuclei (as protons only made half), leading to the modern atomic model where neutrons stabilise the nucleus by counteracting proton repulsion.
What was Chadwick’s model determined from his research?
. Didn’t propose a new atomic model himself, but contributed to refining the modern nuclear model of the atom
. His discovery also provided crucial support for the Quantum Mechanical Model of the Atom
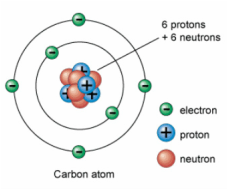
How did Chadwick’s model improve the understanding of the structure of the atom?
. Provided proof for the existence of the neutron
. before Chadwick, the atomic nucleus was thought to contain only protons, but this didn’t explain the difference between atomic number and atomic mass, so Chadwick’s discovery showed that the nucleus consists of both protons (forming atomic number and half of atomic mass) and neutrons (forming the other half of atomic mass), solving this
. If the nucleus contained only protons, their positive charges would strongly repel each other, however neutrons were now known to act as a nuclear glue, reducing repulsion between protons and stabilising the atom
What previous model did Chadwick improve on?
. Improved on Rutherford’s nuclear model of the atom (that proposed the nuclear model with a dense, positively charged nucleus and electrons orbiting around it), and Bohr’s planetary model (which refined Rutherford’s model by introducing quantised electron energy levels, explaining atomic spectra)
. The Rutherford-Bohr model described the nucleus as containing only protons, which couldn’t fully explain atomic mass - neutron discovery explained that neutrons made half of mass and protons made half
. Previous model also didn’t explain how the nucleus remained stable despite the repulsion between positively charged protons - Chadwick explained nuclear stability as neutrons acted as a nuclear glue, reducing repulsion between protons + making nucleus stable
What model superseded Chadwick’s model?
. The model that superseded Chadwick’s contribution was the Quantum Mechanical model of the atom, developed by Erwin Schrodinger
Timeline summary on the history of the atom
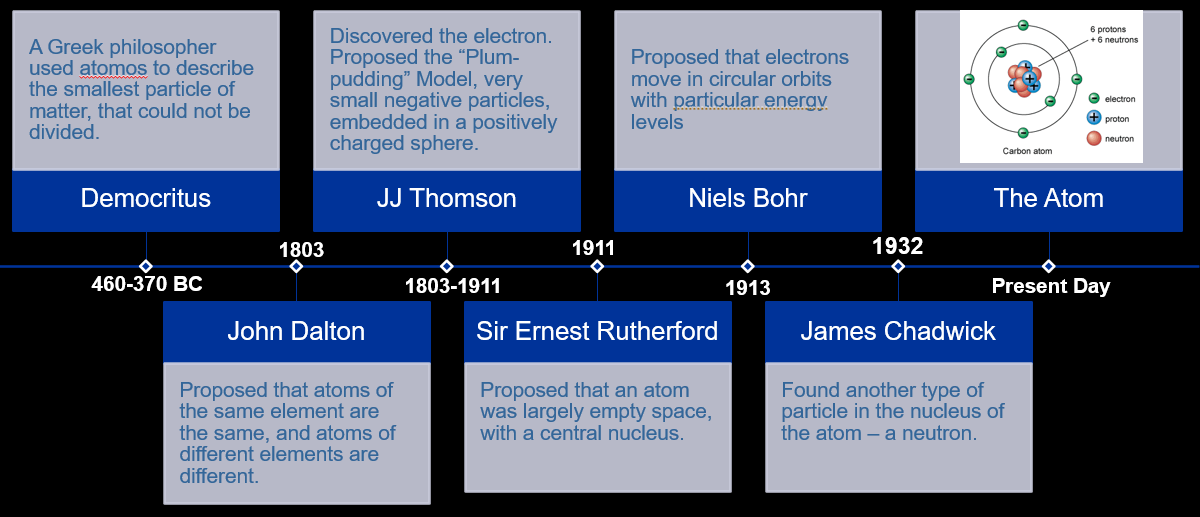
Summary of what each scientist discovered for the atom question