physical science 2025 thermodynamics
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
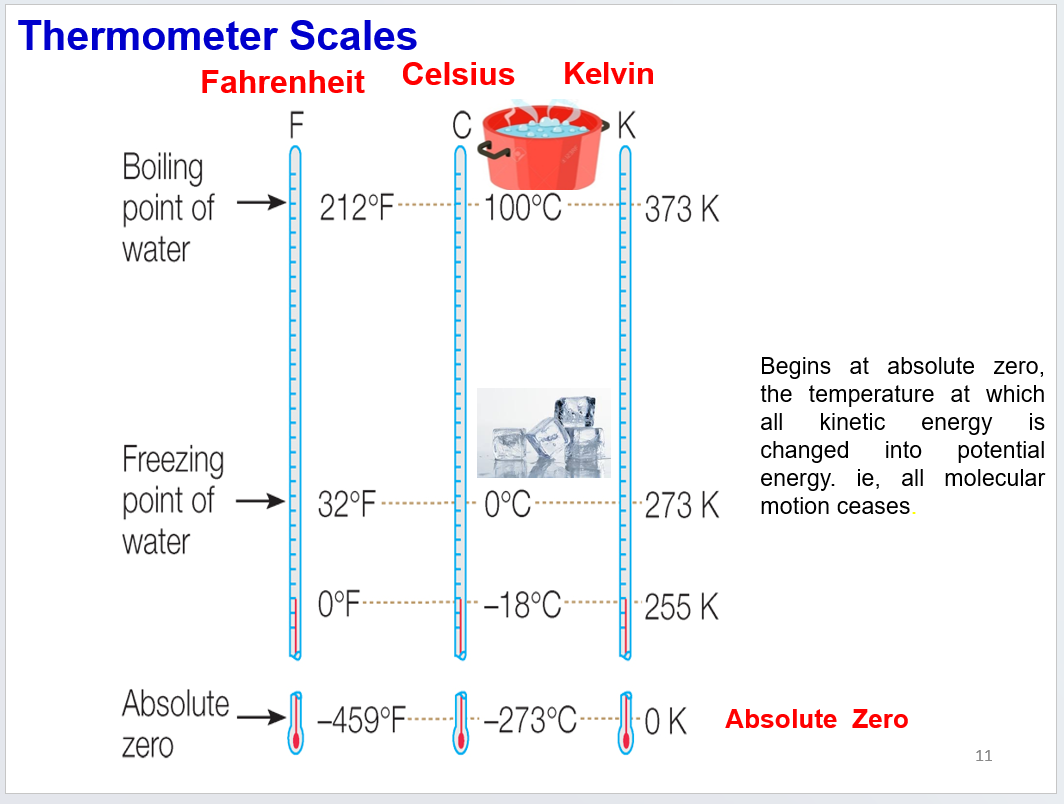
know the boiling, freezing point, and absolute zero
heat:
External energy is total potential and kinetic energy of everyday sized objects. External energy is the kinetic and potential energy that you can see.
Heat : it is the internal ENERGY is the total kinetic and potential energy of an object molecules.
3 ways to transfer heat
conduction: energy transfer from molecule to molecule, hand touching hot pot
convection: molecules with higher kinetic energy move from one place to another. only happens in liquids and gases. water boiling in a kettle cooker. hot water boiling turning and moving up to cooler air
radiation: energy transfer by electromagnetic radiation. campfire heat feeling it by hand. also by visible light and light rays
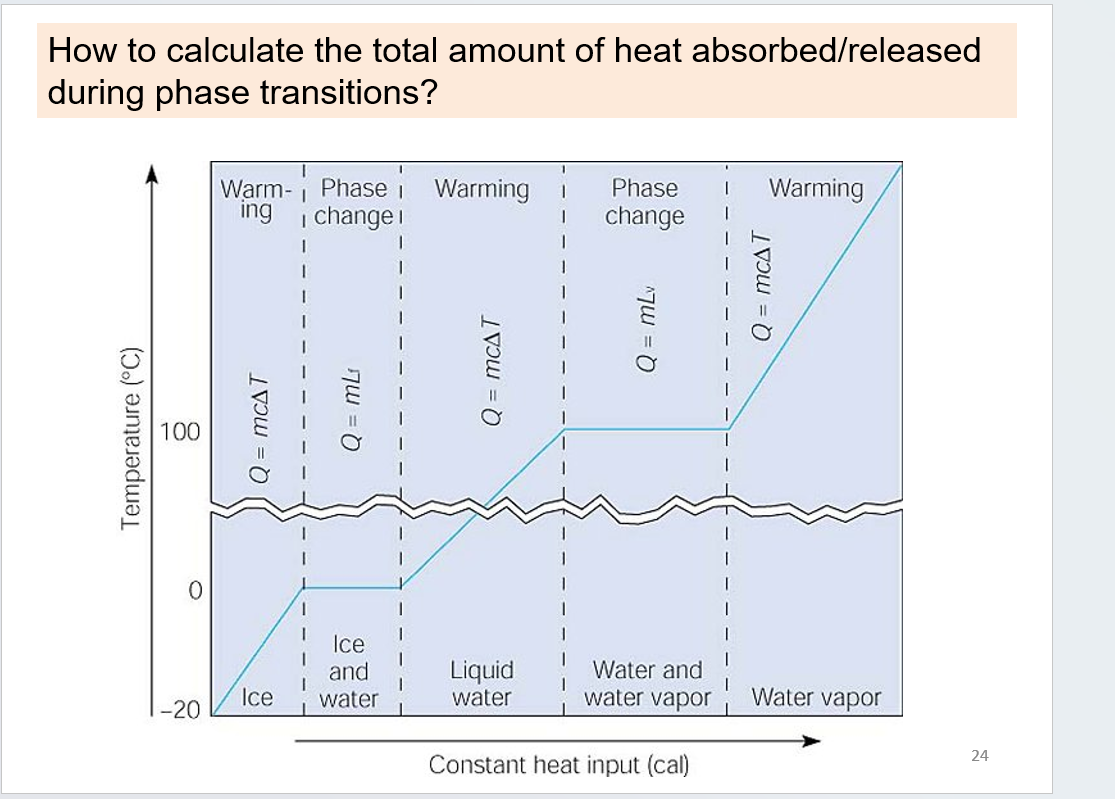
energy heat and molecular theory: phase change
3 ways motion of a molecule can be increased by?
adding heat
absorption of one of the forms of energy
temperature increase to the specific heat of the material
latent heat of fusion (Lf) heat changing a solld to liquid phase of melting or freezing
latent heat of vaporization (Lv): heat invovled during a phase change from a liquid to a gas
1st law of thermodynamics: between any 2 states, the change in internal energy is equal to the difference of heat transfer to the system and work done by the system
2nd law of therymodynamics: heat flows from objects with a higher temperature to an object with a cooler one. work is needed to move the energy from cooler to higher temperature