BCH4053 Exam 4 (Summer 2022)
0.0(0)
0.0(0)
Card Sorting
1/155
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
156 Terms
1
New cards
Where does the citric acid (TCA) cycle occur?
Mitochondria
2
New cards
What does the citric acid cycle produce per 1 molecule of Acetyl-CoA?
* 3 NADH
* 1 FADH2
* 1 GTP
* 2 CO2
* 1 FADH2
* 1 GTP
* 2 CO2
3
New cards
What is the committed step of the citric acid cycle?
Formation of citrate
(Acetyl CoA undergoes condensation rxn with oxaloacetate to form citrate)
(Acetyl CoA undergoes condensation rxn with oxaloacetate to form citrate)
4
New cards
Where do the molecules produced in the citric acid cycle go?
Electron Transport Chain (ETC)
5
New cards
What can Acetyl CoA be formed from?
Pyruvate
6
New cards
How do Acetyl CoA form?
Pyruvate is produced by glycolysis in the cytoplasm. It is transported to the mitochondria where it undergoes oxidative decarboxylation and is converted to Acetyl CoA via the enzyme Pyruvate Dehydrogenase. This conversion is also accompanied by the reduction of NAD+ to NADH.
7
New cards
The Citric Acid Cycle
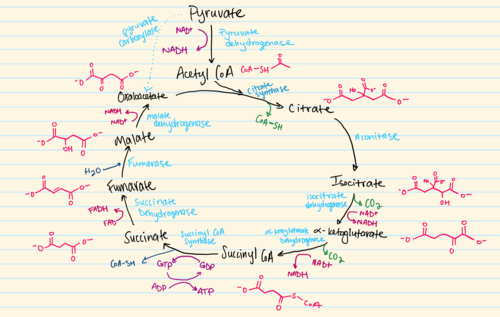
8
New cards
What does a large negative delta G indicate?
Tells us the reaction is highly exergonic and highly favorable. This will indicate there needs to be regulation of some sort at these given steps.
9
New cards
Which reactions of the TCA cycle produce NADH?
Reactions 3, 4, and 8
(isocitrate --> alpha-ketoglutarate)
(alpha-ketoglutarate --> succinyl CoA)
(malate --> oxaloacetate)
NADH is used for ETC
(isocitrate --> alpha-ketoglutarate)
(alpha-ketoglutarate --> succinyl CoA)
(malate --> oxaloacetate)
NADH is used for ETC
10
New cards
Which reaction of the TCA cycle produce FADH2?
Reaction 6
(succinate --> fumarate)
(succinate --> fumarate)
11
New cards
Which reactions of the TCA cycle use H2O as a reactant?
Reactions 1 and 7
(acetyl CoA + oxaloacetate --> citrate)
(fumarate --> malate)
(acetyl CoA + oxaloacetate --> citrate)
(fumarate --> malate)
12
New cards
Which reaction of the TCA cycle produces GTP?
Reaction 5 produces GTP
(succinyl CoA --> succinate)
(succinyl CoA --> succinate)
13
New cards
How does the oxidation of 1 glucose molecule into 2 pyruvate molecules form 8 ATP equivalents?
1 glucose is oxidized to 2 pyruvate which makes 2 ATP. 2 NADH makes 6 ATP from Glycolysis (8 total).
14
New cards
How does the conversion of 2 pyruvate into 2 Acetyl CoA form 6 ATP equivalents?
1 molecule of NAD+ is reduced to NADH when pyruvate is converted into Acetyl CoA. 1 NAD+ gives 3 ATP, since there are 2 NAD+ it makes six ATP total when oxidized.
15
New cards
How does the oxidation of 2 Acetyl CoA in the TCA cycle produce 24 ATP equivalents?
* 12 ATP equivalents are formed from the complete oxidation of one Acetyl-CoA. Since there are 2 pyruvate being converted to 2 Acetyl CoA, there will be 24 ATP total.
* 3 NADH = 9 ATP x 2 = 18 ATP
* 1 FADH2 = 2 ATP x 2 = 4 ATP
* 1 GTP = 1 ATP x 2 = 2 ATP
Total: 24 ATP
* 3 NADH = 9 ATP x 2 = 18 ATP
* 1 FADH2 = 2 ATP x 2 = 4 ATP
* 1 GTP = 1 ATP x 2 = 2 ATP
Total: 24 ATP
16
New cards
How many total ATP equivalents are formed from the complete oxidation of one glucose molecule?
Up to 34
17
New cards
What are anaplerotic (filling up) reactions?
Reactions providing intermediates to the TCA cycle that are not oxaloacetate
18
New cards
What determines the rate of TCA?
Concentrations of oxaloacetate in the cell determine the rate of TCA
19
New cards
What are the 3 major anaplerotic reactions?
* Pyruvate to Oxaloacetate via pyruvate carboxylase
* PEP to oxaloacetate via PEP carboxylase
* Pyruvate to Malate via malic enzyme
* PEP to oxaloacetate via PEP carboxylase
* Pyruvate to Malate via malic enzyme
20
New cards
Why is PEP carboxykinase not anaplerotic?
It goes the wrong direction
21
New cards
What are the 3 reactions that are the focus of regulation in the TCA cycle?
* Step 1:
Oxaloacetate + Acetyl CoA to Citrate (catalyzed by citrate synthase)
* Step 3:
Isocitrate to a-ketoglutarate + NADH (catalyzed by isocitrate dehydrogenase)
* Step 4:
a-ketoglutarate to Succinyl-CoA (catalyzed by a-ketoglutarate dehydrogenase)
Oxaloacetate + Acetyl CoA to Citrate (catalyzed by citrate synthase)
* Step 3:
Isocitrate to a-ketoglutarate + NADH (catalyzed by isocitrate dehydrogenase)
* Step 4:
a-ketoglutarate to Succinyl-CoA (catalyzed by a-ketoglutarate dehydrogenase)
22
New cards
Why is TCA regulated at multiple points?
* Too fast = energy wasted, buildup of cofactors/coenzymes becomes toxic
* Too slow = not enough energy to function and produce intermediates...might die
* Too slow = not enough energy to function and produce intermediates...might die
23
New cards
What regulates Citrate Synthase?
ATP = inhibit
NADH = inhibit
Succinyl-CoA = inhibit
NADH = inhibit
Succinyl-CoA = inhibit
24
New cards
What regulates Isocitrate Dehydrogenase?
ATP = inhibit
ADP = activate
NAD+ = activate
ADP = activate
NAD+ = activate
25
New cards
What regulates alpha-ketoglutarate dehydrogenase?
NADH = inhibit
(this step produces NADH so if there is enough...inhibit)
Succinyl-CoA = inhibit
(this step produces Succinyl-CoA...doesn't need to produce more if enough already)
AMP = activate
(this step produces NADH so if there is enough...inhibit)
Succinyl-CoA = inhibit
(this step produces Succinyl-CoA...doesn't need to produce more if enough already)
AMP = activate
26
New cards
What is the purpose of the pyruvate dehydrogenase complex?
Converts pyruvate to Acetyl CoA and then determines whether this molecule will enter into the TCA cycle or continue on to Fatty Acid Synthesis
27
New cards
How is pyruvate dehydrogenase (PDH) regulated?
* Covalently via Phosphorylation
* Allosterically
- phosphorylated = inactive
- de-phosphorylated = active
- products inhibit enzyme
- substrates promote enzyme
* High NADH/Acetyl-CoA concentrations allosterically activate PDH to phosphorylate serine 203, 264, 271 on alpha subunit of PDH (blocks initial PDH rxn)
* PDH reactivated by Ca2+ dependent enzyme PDH phosphatase (hydrolyzes phosphoserines)
- Low NADH or high NAD+ or low Acetyl-CoA concentrations activate PDH phosphatase
* Insulin/Ca2+ ions activate PDH and pyruvate blocks phosphorylation
* Allosterically
- phosphorylated = inactive
- de-phosphorylated = active
- products inhibit enzyme
- substrates promote enzyme
* High NADH/Acetyl-CoA concentrations allosterically activate PDH to phosphorylate serine 203, 264, 271 on alpha subunit of PDH (blocks initial PDH rxn)
* PDH reactivated by Ca2+ dependent enzyme PDH phosphatase (hydrolyzes phosphoserines)
- Low NADH or high NAD+ or low Acetyl-CoA concentrations activate PDH phosphatase
* Insulin/Ca2+ ions activate PDH and pyruvate blocks phosphorylation
28
New cards
Describe the basics of the other 3 layers of regulation associated with the pyruvate dehydrogenase complex
* Acetyl CoA blocks dihydrolipoyl transacetylase
* NADH inhibits dihyrolipoyl dehydrogenase
* AMP activates pyruvate dehydrogenase
* GTP inhibits pyruvate dehydrogenase
* NADH inhibits dihyrolipoyl dehydrogenase
* AMP activates pyruvate dehydrogenase
* GTP inhibits pyruvate dehydrogenase
29
New cards
Understand how Lysine Acetylation is a regulatory mechanism that all enzymes under the TCA cycle are held under
* Acetylation inhibits TCA activity
* SIRT3 is mitochondrial NAD+ dependent deacetylase responsible for deacylation and activation of TCA enzymes
* SIRT3 is mitochondrial NAD+ dependent deacetylase responsible for deacylation and activation of TCA enzymes
30
New cards
Discuss SIRT3 expression in the body
* SIRT3 expression increases with exercise
- The more you exercise, the more energy you need (SIRT3 is produced to keep TCA actively producing energy)
* SIRT3 production is linked to longevity in humans
- The more you exercise, the more energy you need (SIRT3 is produced to keep TCA actively producing energy)
* SIRT3 production is linked to longevity in humans
31
New cards
What is the goal of the Electron Transport Chain?
* To use oxidative phosphorylation to convert ADP to ATP
* NADH/FADH2 dependent ATP synthesis
- NADH/FADH2 are oxidized
* NADH/FADH2 dependent ATP synthesis
- NADH/FADH2 are oxidized
32
New cards
How much ATP does 1 glucose yield in the Electron Transport Chain?
34 ATP
33
New cards
Describe key features of the mitochondria
* Outer membrane is porous/permeable to ions and small molecules
* Inner membrane is impermeable and transport requires proteins (all enzymes/proteins required for oxidative phosphorylation are in the inner membrane)
* Inner membrane contains enzymes adenylate kinase and nucleoside-diphosphate kinase (play role in energy metabolism)
* Mitochondrial matrix contains oxidative enzymes, pyruvate dehydrogenase (PDH), and enzymes of TCA and fatty acid (beta) oxidation
* Inner membrane is impermeable and transport requires proteins (all enzymes/proteins required for oxidative phosphorylation are in the inner membrane)
* Inner membrane contains enzymes adenylate kinase and nucleoside-diphosphate kinase (play role in energy metabolism)
* Mitochondrial matrix contains oxidative enzymes, pyruvate dehydrogenase (PDH), and enzymes of TCA and fatty acid (beta) oxidation
34
New cards
Is the electron transport chain aerobic or anaerobic?
Aerobic
35
New cards
What are the 4 complexes involved in the ETC?
* Complex I (NADH CoQ Reductase)
* Complex II (Succinate-CoQ Reductase)
* Complex III (CoQ-Cytochrome C Reductase)
* Complex IV (Cytochrome C Oxidase)
* Complex II (Succinate-CoQ Reductase)
* Complex III (CoQ-Cytochrome C Reductase)
* Complex IV (Cytochrome C Oxidase)
36
New cards
Electron Transport Chain (ETC)
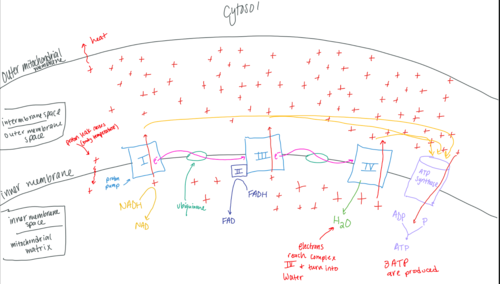
37
New cards
Does Complex I of the ETC pump protons? If so, how many?
4 protons are pumped for every NADH oxidized
38
New cards
Complex I catalyzes the oxidation of _________ and reduction of ____________ .
NADH (oxidized)
Ubiquinone (reduced)
Ubiquinone (reduced)
39
New cards
What carrier carries electrons from Complex I to Complex III
Coenzyme Q (CoQ)
- also called ubiquinone
2 electrons are passed from NADH --> FMNH2 --> Fe-S --> CoQ (ubiquinone)
- also called ubiquinone
2 electrons are passed from NADH --> FMNH2 --> Fe-S --> CoQ (ubiquinone)
40
New cards
Are protons pumped in Complex II of the ETC? If so, how many?
No protons pumped
41
New cards
Complex II of the ETC catalyzes the oxidation of _________ and reduction of __________ .
FADH2 (from TCA) oxidized
Ubiquinone (UQ) reduced to (UQH2)
Ubiquinone (UQ) reduced to (UQH2)
42
New cards
What is succinate oxidized into in Complex II of the ETC?
Fumarate
43
New cards
Complex III of the ETC catalyzes oxidation of __________ and reduction of __________ .
1 UQH2 (oxidized)
2 Cytochrome C molecules (reduced)
2 Cytochrome C molecules (reduced)
44
New cards
Complex III accepts electrons from coenzyme QH2 that is generated from which complex(es)?
Complex II
45
New cards
What cycle is enacted in complex III?
Q Cycle
46
New cards
What is the Q cycle? What does it do?
Mechanism for coupling electron transfer from QH2 to cytochrome c found in Complex III
47
New cards
The iron (Fe) in the cytochrome proteins is located in a porphyrin-ring structure (like heme), and can exist in what two oxidation states?
Fe2+ and Fe3+
48
New cards
Are protons pumped in Complex IV? If so, how many?
4 protons are transported across the inner membrane of the mitochondria
49
New cards
Electrons combine with ________ and _______ to form 2 molecules of water
Cytochrome C Oxidase accepts electrons from reduced Cytochrome C (complex III) and used them to reduce molecular oxygen (O2) to water (H2O)
50
New cards
Explain proton/electron movement in Complex IV of the ETC
For every 4 electrons passing through Complex IV, 8 protons are removed from the matrix side of the membrane
(4 protons are transferred to intermembrane space, other 4 protons end up in water molecules)
(4 protons are transferred to intermembrane space, other 4 protons end up in water molecules)
51
New cards
How is the proton motor force established?
The differences the protons make both in concentration and electrostatic gradients across the membrane can be related to free energy
52
New cards
Is the export of protons favorable or unfavorable?
Unfavorable
(export of protons has has a positive free energy change)
(export of protons has has a positive free energy change)
53
New cards
By default, is the pulling of protons across the membrane favorable or unfavorable?
Favorable
(Delta G = -23.3 kJ/mol thus it IS favorable)
(Delta G = -23.3 kJ/mol thus it IS favorable)
54
New cards
What is the ATP Synthase?
The enzyme complex that carries out ATP synthesis in the mitochondria
55
New cards
What is another name for ATP Synthase?
F1-F0-ATPase
56
New cards
What are the two parts that make up the ATP Synthase?
F1 and F0
57
New cards
What is the F1 complex of ATP Synthase responsible for?
* F1 performs the ATP synthesis activity
* F1 protrudes into the matrix environment
* Contains 5 subunits (a,b,g,d,e)
* F1 complex's 'b' subunit is the location of synthesis activity
* c,g,e rotate as protons flow through (rotor)
* F1 protrudes into the matrix environment
* Contains 5 subunits (a,b,g,d,e)
* F1 complex's 'b' subunit is the location of synthesis activity
* c,g,e rotate as protons flow through (rotor)
58
New cards
What is the F0 complex of ATP Synthase responsible for?
* F0 is designed for the flow of protons (embedded in membrane)
- rotates counterclockwise
* F0 has 3 subunits (a,b,c)
* Has transmembrane pore from the intermembrane space to the matrix
- rotates counterclockwise
* F0 has 3 subunits (a,b,c)
* Has transmembrane pore from the intermembrane space to the matrix
59
New cards
Describe the 3 conformations the ATP Synthase goes through while rotating
OPEN
- low affinity for ATP
- not catalytically active
LOOSE
- loosely binds ATP, ADP, phosphate ions
- not catalytically active
TIGHT
- tightly binds ATP, ADP, phosphate ions
- catalytically active
- low affinity for ATP
- not catalytically active
LOOSE
- loosely binds ATP, ADP, phosphate ions
- not catalytically active
TIGHT
- tightly binds ATP, ADP, phosphate ions
- catalytically active
60
New cards
Which direction does the c-subunit of F0 of ATP Synthase rotate? Why?
Counterclockwise because it is energetically favorable and there are no repulsive interactions between the amino acids that make up the subunit and the protons being pumped from the highly concentrated intermembrane space to the less concentrated matrix
61
New cards
How many protons (H+) are required to make 1 ATP?
3 protons
62
New cards
How many protons (H+) are made from 1 NADH?
10 protons
63
New cards
How many ATP can form from 1 NADH?
1 NADH produces 10 protons
If 3 protons make up 1 ATP, then
10/3 = 3.33 ATP
If 3 protons make up 1 ATP, then
10/3 = 3.33 ATP
64
New cards
How does Rotenone inhibit the ETC?
Inhibits NADH-UQ Reductase in Complex I
65
New cards
How do Barbiturates inhibit the ETC?
Inhibit NADH-UQ Reductase in Complex I
66
New cards
How does Demerol inhibit the ETC?
Inhibits NADH-UQ Reductase in Complex I
(Demerol is a pain killer)
(Demerol is a pain killer)
67
New cards
How does Cyanide inhibit the ETC?
Binds tightly to the ferric form of Cytochrome A in Complex IV
68
New cards
How does Azide inhibit the ETC?
Binds tightly to the ferric form of Cytochrome A in Complex IV
69
New cards
How does Carbon Monoxide inhibit the ETC?
Binds tightly to the ferric form of Cytochrome A in Complex IV
70
New cards
How does Oligomycin inhibit the ETC?
Blocks movement of protons through the F0 complex of ATP Synthase
71
New cards
What do uncouplers do?
* Dissipate proton gradient across inner mitochondrial membrane
- Combine with cytosolic protons and carry them back into the matrix
(Uncouplers produce heat/release energy from the uncoupled flow of protons through the membrane...a mechanism by which an organism can warm itself up when needed)
(ATP Synthase needs proton gradient to fxn...uncouplers undo gradient)
- Combine with cytosolic protons and carry them back into the matrix
(Uncouplers produce heat/release energy from the uncoupled flow of protons through the membrane...a mechanism by which an organism can warm itself up when needed)
(ATP Synthase needs proton gradient to fxn...uncouplers undo gradient)
72
New cards
What are physical properties of uncouplers?
They are hydrophobic molecules with functional groups that can be protonated or deprotonated
73
New cards
What are 2 examples of uncouplers?
2,4-Dinitrophenol and Dicumarol
74
New cards
What is ATP-ADP Translocase?
An enzyme that moves ATP from the mitochondria to other parts of the cell for use and brings ADP back into the mitochondria for "recharging"
75
New cards
Describe how ATP-ADP Translocase works?
The enzyme transfers 1 ADP into the mitochondria for every 1 ATP that is transferred out
(the charge difference (1-) between ATP (4-) and ADP (3-) as well as the concentration gradient makes these transfers spontaneous)
(an additional proton must be used to neutralize (1-) charge, thus total cost of protons to 1 ATP production is 4:1)
(the charge difference (1-) between ATP (4-) and ADP (3-) as well as the concentration gradient makes these transfers spontaneous)
(an additional proton must be used to neutralize (1-) charge, thus total cost of protons to 1 ATP production is 4:1)
76
New cards
What is the P/O Ratio? (Phosphate/Oxygen Ratio)
The ratio between the number of molecules of ATP formed in oxidative phosphorylation for every 2 electrons flowing through a defined segment of the ETC
77
New cards
What does the P/O Ratio assume?
Assume 10 protons out of matrix for every 2 electrons passing through ETC, and 4 protons transported from cytosol into matrix
(~ 2.5 molecules of ATP per 2 electrons flowing)
(~ 2.5 molecules of ATP per 2 electrons flowing)
78
New cards
List the steps of the Glycerophosphate shuttle
1. Electrons in NADH are transferred to DHAP to produce G-3-P and NAD+
2. G-3-P transfers its electrons directly to FAD in the inner mitochondrial membrane producing FADH2
3. FADH2 proceeds through the ETC
2. G-3-P transfers its electrons directly to FAD in the inner mitochondrial membrane producing FADH2
3. FADH2 proceeds through the ETC
79
New cards
How much ATP is created per glucose molecule through the Glycerophosphate shuttle?
32 ATP
80
New cards
Is the malate aspartate shuttle reversible or irreversible?
Reversible
81
New cards
List the steps of the malate aspartate shuttle
1. NADH oxidized in cytosol by reducing Oxaloacetate to Malate
2. Electrons brought into matrix as Malate is transported into matrix by a-ketoglutarate-malate transporter
3. Malate converted back into Oxaloacetate by Malate Dehydrogenase with concurrent reduction of NAD+ to NADH
4. Oxaloacetate is transaminated to Aspartate by the Aspartate Aminotransferase
5. Aspartate and Glutamate swap sides of membrane via Aspartate-Glutamate Carrier
(no charge difference, thus requires no additional protons...more efficient than glycerophosphate shuttle)
2. Electrons brought into matrix as Malate is transported into matrix by a-ketoglutarate-malate transporter
3. Malate converted back into Oxaloacetate by Malate Dehydrogenase with concurrent reduction of NAD+ to NADH
4. Oxaloacetate is transaminated to Aspartate by the Aspartate Aminotransferase
5. Aspartate and Glutamate swap sides of membrane via Aspartate-Glutamate Carrier
(no charge difference, thus requires no additional protons...more efficient than glycerophosphate shuttle)
82
New cards
How many ATP are made per glucose if the malate-aspartate shuttle is used?
34 ATP
83
New cards
What is the goal of gluconeogenesis?
To create glucose from non-carbohydrate precursors
84
New cards
What are the non-carbohydrate precursors of gluconeogenesis?
Pyruvate, all amino acids (except lysine/leucine), lactic acid, glycerol, and any TCA intermediate
85
New cards
How many steps of Gluconeogenesis are retained from Glycolysis? Which ones?
7 steps retained
(steps 2 and 4-9)
(steps 2 and 4-9)
86
New cards
What are the 3 steps of Glycolysis replaced in Gluconeogenesis?
Steps 1, 3, and 10
(pyruvate carboxylase, PEP carboxylase, fructose-1,6-bisphosphatase, glucose 6 phosphatase)
(pyruvate carboxylase, PEP carboxylase, fructose-1,6-bisphosphatase, glucose 6 phosphatase)
87
New cards
What do the new enzymes of gluconeogenesis replace in glycolysis?
* Pyruvate carboxylase and PEP carboxykinase replace pyruvate kinase
* Fructose-1,6-bisphosphatase replaces phosphofructokinase
* Glucose-6-phosphatase replaces hexokinase
(These new rxns provide for spontaneous pathway in reverse direction of glycolysis...also provides new mechanism of regulation)
* Fructose-1,6-bisphosphatase replaces phosphofructokinase
* Glucose-6-phosphatase replaces hexokinase
(These new rxns provide for spontaneous pathway in reverse direction of glycolysis...also provides new mechanism of regulation)
88
New cards
Where does gluconeogenesis occur?
Mainly in the liver and kidneys
89
New cards
What does Pyruvate Carboxylase do and how is it regulated?
Converts Pyruvate to Oxaloacetate (uses ATP and CO2)
* Allosterically activated by Acetyl-CoA (body puts oxaloacetate and pyruvate into gluconeogenesis)
* Acetyl CoA levels low = body pushes pyruvate and oxaloacetate into TCA and glycolysis
* Allosterically activated by Acetyl-CoA (body puts oxaloacetate and pyruvate into gluconeogenesis)
* Acetyl CoA levels low = body pushes pyruvate and oxaloacetate into TCA and glycolysis
90
New cards
What does PEP carboxykinase do?
Converts oxaloacetate to PEP (uses GTP; makes CO2)
91
New cards
What does Fructose-1,6-bisphosphatase do?
Hydrolyzes fructose-1,6-bisphosphate to fructose-6-phosphate
92
New cards
What is the reaction for pyruvate carboxylase?
pyruvate + bicarbonate + ATP --> oxaloacetate + ADP + Pi
This enzyme is Biotin dependent (bicarbonate is the biotin)
This enzyme is Biotin dependent (bicarbonate is the biotin)
93
New cards
What regulates Fructose-1,6-bisphosphatase?
* Citrate stimulates
* Fructose-2,6-bisphosphate inhibits
* AMP inhibits
* Fructose-2,6-bisphosphate inhibits
* AMP inhibits
94
New cards
What is the reaction for fructose-1,6-bisphosphatase?
F-1,6-BP + H2O --> F-6-P + Pi
95
New cards
What does Glucose-6-phosphatase do?
Converts G-6-P to Glucose in the Endoplasmic Reticulum
96
New cards
Explain the Glucose-6-Phosphatase system
The glucose-6-phosphatase system includes phosphatase itself and 3 transport proteins (T1, T2, T3)
T1 takes G-6-P into the ER where it is hydrolyzed by the phosphatase
T2 and T3 export glucose and Pi, respectively, to the cytosol
GLUT2 exports glucose to the circulation
T1 takes G-6-P into the ER where it is hydrolyzed by the phosphatase
T2 and T3 export glucose and Pi, respectively, to the cytosol
GLUT2 exports glucose to the circulation
97
New cards
Explain 2 reasons why gluconeogenesis is not the mere reversal of glycolysis
1. If the processes were reversible there would be no room for regulation which is absolutely necessary (glycolysis on, gluconeogenesis off...vice versa)
2. Glycolysis has delta G of -74 kJ/mol...if gluconeogenesis were simply the reverse it would have a positive delta G which would make the reaction impossible
2. Glycolysis has delta G of -74 kJ/mol...if gluconeogenesis were simply the reverse it would have a positive delta G which would make the reaction impossible
98
New cards
Why does gluconeogenesis only occur in the liver and kidneys?
Brain and muscles lack enzymes to allow gluconeogenesis to occur
99
New cards
Explain the Cori Cycle
* Lactate is produced in the muscle during exercise. Lactate buildup causes cramping due to lack of oxygen
* NADH reoxidized during reduction of pyruvate to lactate
* Lactate returns to liver where it can be reoxidized to pyruvate by lactate dehydrogenase
* Liver produces/provides glucose to muscle for exercise and then reprocesses lactate into new glucose
* NADH reoxidized during reduction of pyruvate to lactate
* Lactate returns to liver where it can be reoxidized to pyruvate by lactate dehydrogenase
* Liver produces/provides glucose to muscle for exercise and then reprocesses lactate into new glucose
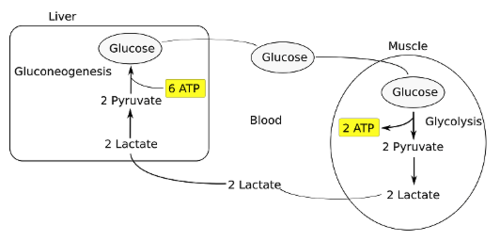
100
New cards
What does it mean that gluconeogenesis and glycolysis are under reciprocal control?
Glycolysis is inhibited when gluconeogenesis is running and vice versa