Introduction-[Facilities and Function in a Manufacturing Laboratory Facility]
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
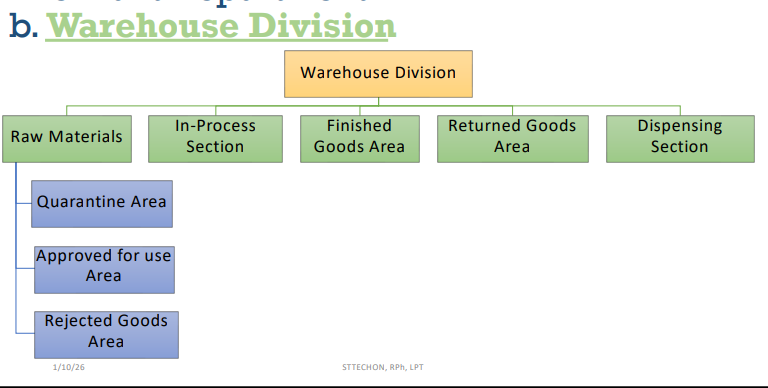
Raw Materials
In-Process Section
Finished Goods Area
Returned Goods Area
The Plant Department:
Warehouse Division [4]
Quarantined Materials
[Raw Materials Section]
Yellow coded labels
Quarantined Materials
[Raw Materials Section]
Materials subject to tests and assays by quality control and not yet to be used.
Approved for Use Materials
WAREHOUSE DIVISION —> [Raw Materials Section]
Green coded labels
Approved for Use Materials
[Raw Materials Section]
Materials that conform to standards and specifications.
Rejected Materials
[Raw Materials Section]
Red coded stickers
Rejected Materials
[Raw Materials Section]
____-
Materials found to be substandard.
These are either returned to the supplier or disposed of properly.
Primary Packaging
What is the type of packaging of In Process Section ?
In Process Section
[Warehouse Division]
Contains products that have been bottled, stripped, or packed but not yet labelled or placed into final boxes or cartons.
In Process Section
[Warehouse Division]
This section is under Quarantine.
Secondary Packaging
What is the type of packaging of Finished Products/Goods?
Goods
Finished Product is also known as ___ ?
Finished Products/Goods
[Warehouse Division]
Contains products that are packaged and finished and are ready for distribution and sale.
Finished Products/Goods
[Warehouse Division]
Contains products that are ready for distribution and sale.
Returned Goods Section
[Warehouse Division]
The products returned are stored in this section of the warehouse.
Returned Goods Section
[Warehouse Division]
Rejected in-process section.
Dispensing Section
[Warehouse Division]
Area after approved for use where raw materials for production are weighed and/or measured.
Dispensing Section
[Warehouse Division]
Packaging materials are also counted upon issuance.
Engineering and Maintenance Section
[Plant Department]
Takes charge of the care, maintenance, and repair of all machines and equipment used in the plant department, including electrical lines, water and sewerage systems, waste management, telecommunication lines, and environmental sanitation, etc.
Calibration of machines.
Works with validators.
Pharmacists
Personnel in Manufacturing:
In-charge with
compounding
dispensing
Quality Assurance (QA
validation
Chemists
Personnel in Manufacturing:
In-charge with Quality Control (QC).
Microbiologists
Personnel in Manufac
In-charge with turing:microbial section.
Industrial Engineers
Personnel in Manufacturing:
In-charge with packaging.
Preparation of Master Formula (MF)
Preparation of the Master formula if the master formula is not the actual amount to be manufactured.
Preparation of Manufacturing Order (MO) and Finishing order
Weighs/measures the ingredients, counts the packaging materials and transfer them to the manufacturing and packaging area respectively.
Compounds the product, according to the standard compounding procedures
While the product is processed, it undergoes in process tests.
The compounded product is transferred to the packaging section where it is bottled/stripped or packed.
Transferring the bottled, stripped or packed products to the In-Process Area of the warehouse
If the in-process tests are approved by the Quality control, the bottled/stripped/packed products are labelled, packed into unit boxes then to cartons by the Packaging section.
The finished products are transferred in the finished goods area of the warehouse ready for distribution and sale.
[Plant Department]
The general procedure for the manufacture of a product is as follows
Research Department (locally)
Parent Company (abroad)
Manufacturing of Pharmaceuticals (Production):
Preparation of Master Formula (MF)
In charged Personnel:
____ [2]
Name of the Product
Potency of the AI
Batch Size, Amount Yield
List of Ingredients and specifications, including code number
Quantity of each ingredient
Signature of the competent people who prepared the MF
Manufacturing of Pharmaceuticals (Production):
The Master Formula consists of the following information:
Quality Control Head — revise MF
Manufacturing of Pharmaceuticals (Production):
Preparation of the Master formula if the master formula is not the actual amount to be manufactured
In Charged Personnel: ______ ?
Production Control (specifically the “planning and scheduling”)
Manufacturing of Pharmaceuticals (Production):
Preparation of Manufacturing Order (MO) and Finishing order.
In charged Personnel: ________ ?
Pharmacist in Dispensing Area
Has the Master Formula (MF), Manufacturing Order (MO), and Filling Order (FO).
Manufacturing of Pharmaceuticals (Production):
Dispenses raw and packaging materials according to these documents.
In charged Personnel: ______ ?
Compounding Pharmacist
Manufacturing of Pharmaceuticals (Production):
Compounds the product, according to the standard compounding procedures
In charged Personnel:_______ ?
Quality Control
Manufacturing of Pharmaceuticals (Production):
While the product is processed, it undergoes in process tests.
In charged Personnel:______ ?
Packaging Section
Manufacturing of Pharmaceuticals (Production):
The compounded product is transferred to the packaging section where it is bottled/stripped or packed.
Transferring the bottled, stripped or packed products to the In-Process Area of the warehouse
In charged Personnel: _______ ?
Quality Control
Manufacturing of Pharmaceuticals (Production):
If the in-process tests are approved by ______, the bottled/stripped/packed products are labelled, packed into unit boxes, then into cartonsby the Packaging section
Packaging section
Manufacturing of Pharmaceuticals (Production):
If the in-process tests are approved by Quality Control, the bottled/stripped/packed products are labelled, packed into unit boxes, then into cartons by the ______?
finished goods area
Manufacturing of Pharmaceuticals (Production):
The finished products are transferred in the_____of the warehouse ready for distribution and sale.
distribution and sale
Manufacturing of Pharmaceuticals (Production):
The finished products are transferred in the finished goods area of the warehouse ready for_________?