Chemistry A Level OCR A; Enthalpy, Entropy and Redox and Electrode potentials
1/164
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
165 Terms
What is the definition if an ionic bond (you have to know this, recap from AS level)?
A strong electrostatic attraction between oppositely charged ions forming a giant ionic lattice structure. Can only occur between a metal and non-metal
Why are ionic bonds stable?
As lots of energy is required to overcome the giant ionic lattice structure's substantial energy barrier
Is Lattice enthalpy (LE) exothermic or endothermic?
Exothermic
What is lattice enthalpy?
The enthalpy change that accompanies the formation of one mole of an ionic compound from its gaseous ions under standard conditions
∆ₗₑH⦵
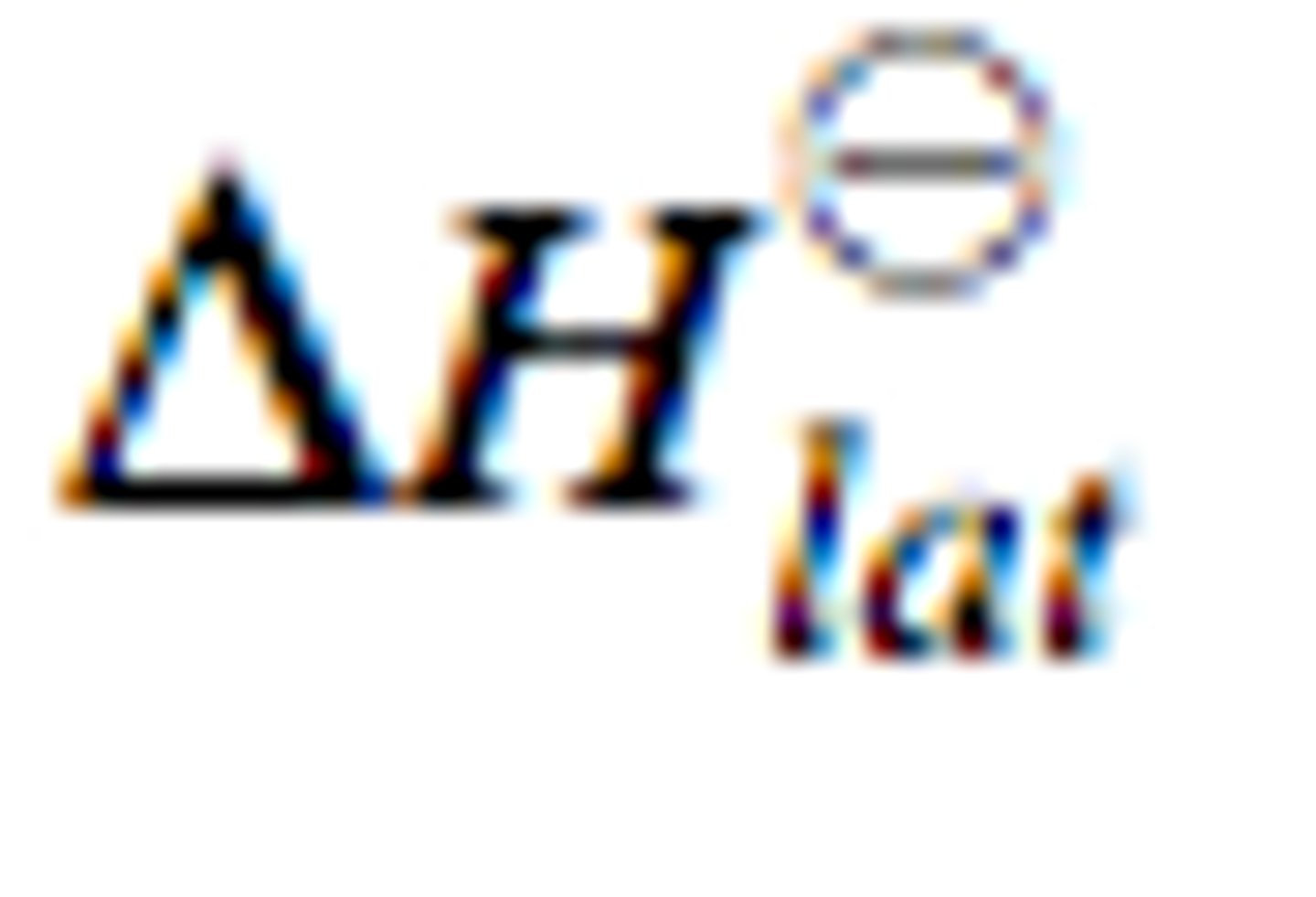
Why is LE exothermic?
As it involves bond formation
As LE is exothermic, what will the value for enthalpy change always be, positive or negative?
Negative (exothermic)
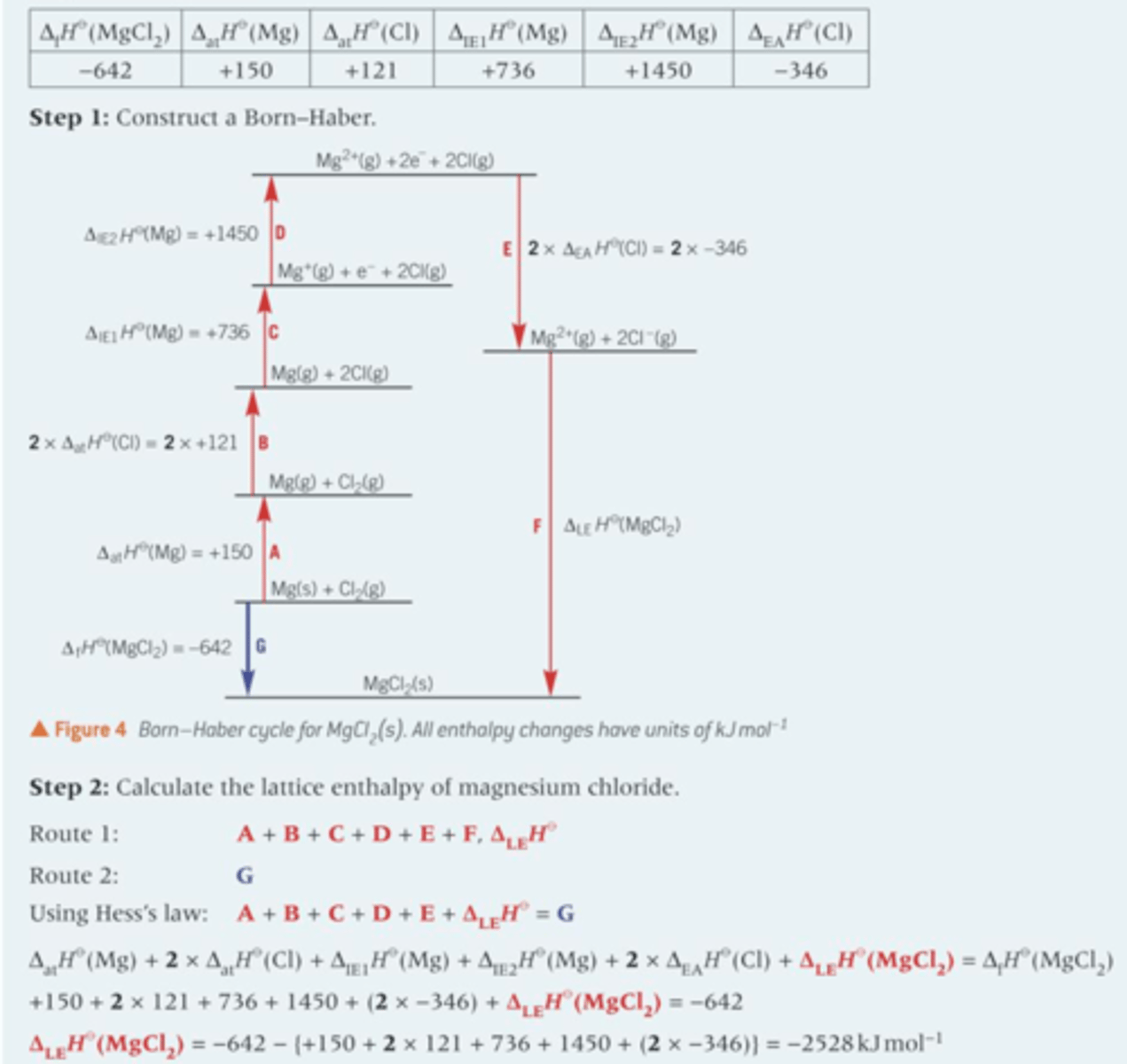
Show the Born-Haber cycle
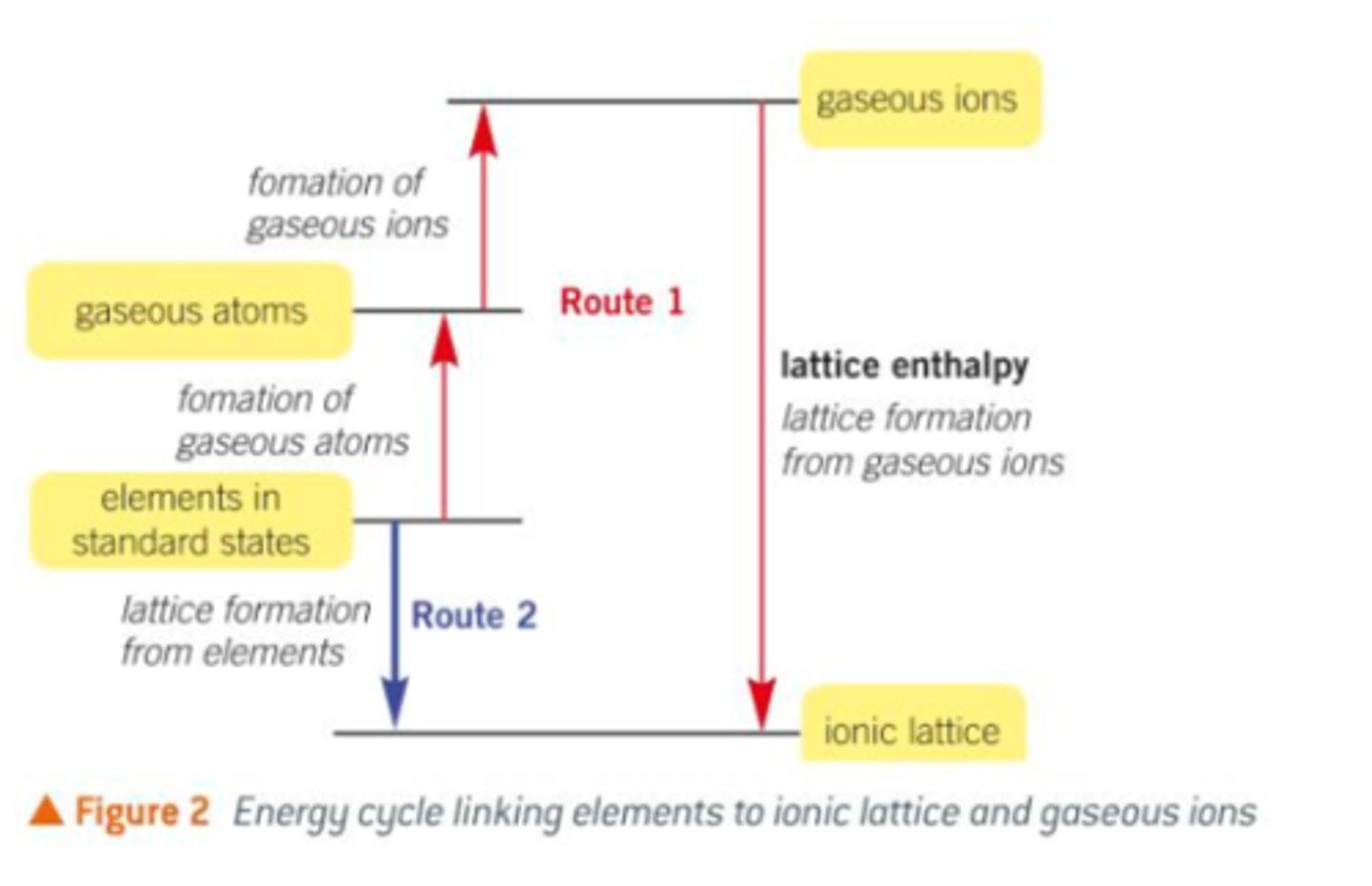
Describe route 1 processes
1) Formation of gaseous atoms from elements in their standard states (Endothermic)
2) Formation of gaseous ions from gaseous atoms (Overall, is endothermic)
3) Lattice formation, changing gaseous ions into their solid ionic lattice (exothermic)
Describe the route 2 process
1) Convert the elements in their standard states directly to the ionic lattice
2) Only involves one enthalpy change, enthalpy change of formation (exothermic)
What are the key enthalpy changes in a full Born-Haber cycle?
- Standard enthalpy change of formation
- Standard enthalpy change of atomisation
- First ionisation energy (Not all the time however there can be a second ionisation energy depending on the ion)
- First electron affinity (Not all the time however there can be a second electron affinity depending on the ion)
What is the standard enthalpy change of formation? (Recap from AS, you must know this off by heart by now)
Enthalpy change that takes place when one mole of a compound is formed from its elements under standard conditions, with all reactants and products in their standard states
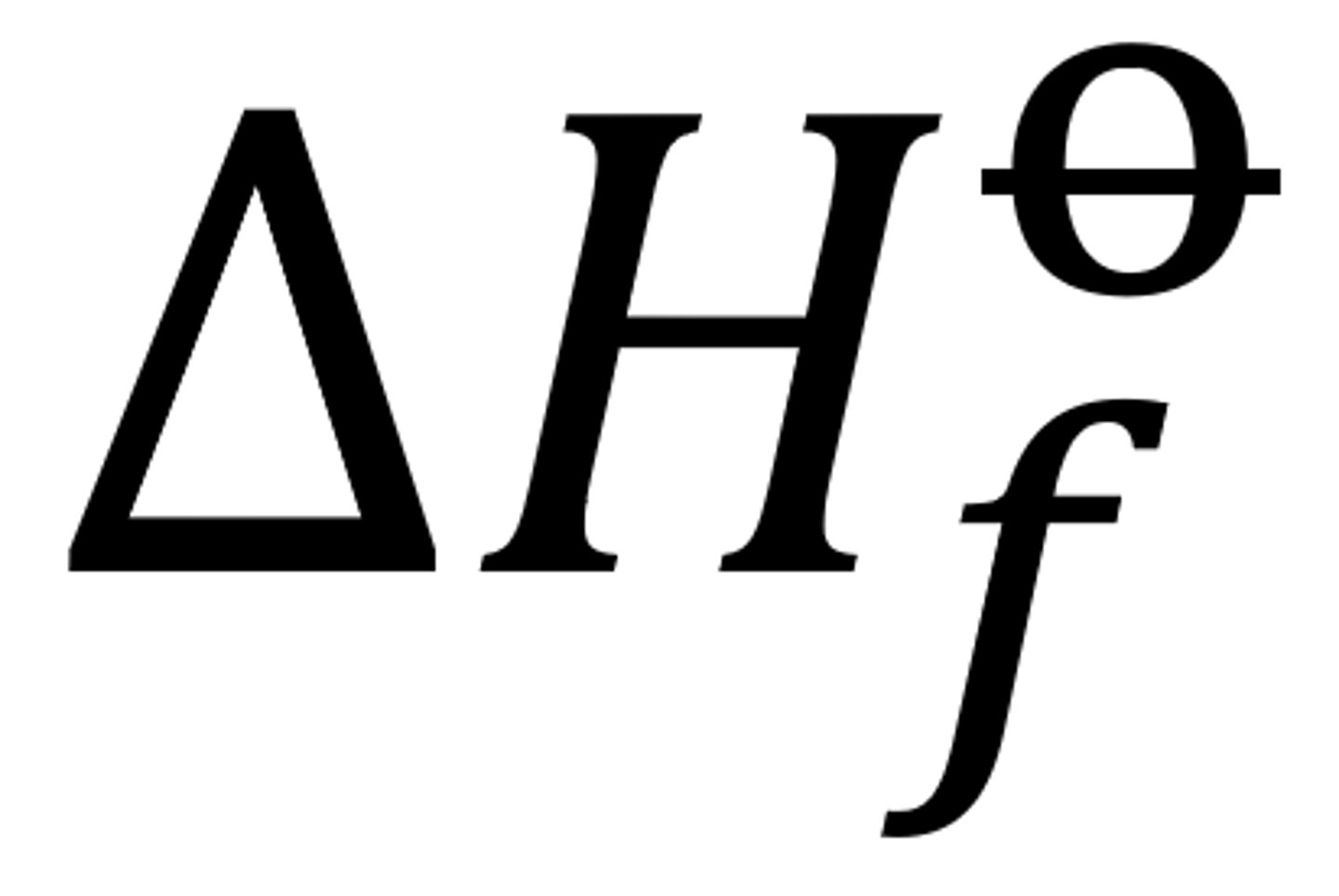
What is the standard enthalpy change of atomisation?
Enthalpy change that takes place for the formation of one mole of gaseous atoms from it's element under standard conditions
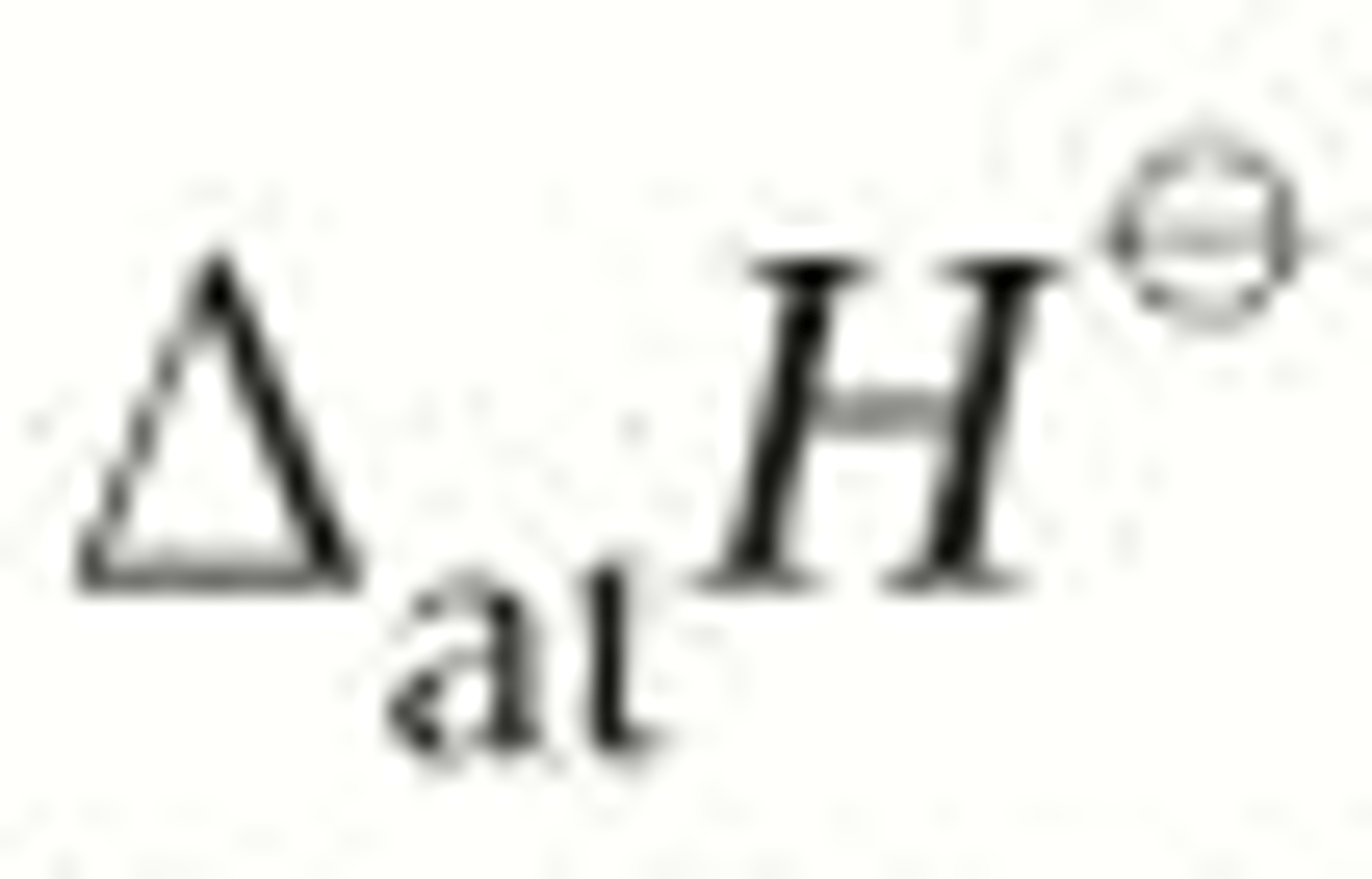
Is standard enthalpy change of atomisation endothermic or exothermic?
Endothermic, always
Why is standard enthalpy change of atomisation always endothermic?
As bonds are being broken to form gaseous atoms
What is the (enthalpy change of the) first ionisation energy?
Enthalpy change that takes place when one mole of electrons are removed from one mole of gaseous atoms to form one mole of gaseous 1+ ions
∆ᵢₑH⦵ (capital IE instead of ie)
Is (standard enthalpy change of) first ionisation energy endothermic or exothermic?
Endothermic, always
Why is (standard enthalpy change of) first ionisation energy always endothermic?
As energy is required to overcome the attraction between a negative electron and the positive nuclei
What is electron affinity? The same or opposite of ionisation energy?
Electron affinity is the opposite of ionisation energy
What is the definition of (the standard enthalpy change of) the first electron affinity?
Enthalpy change that takes place when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous 1- ions under standard conditions
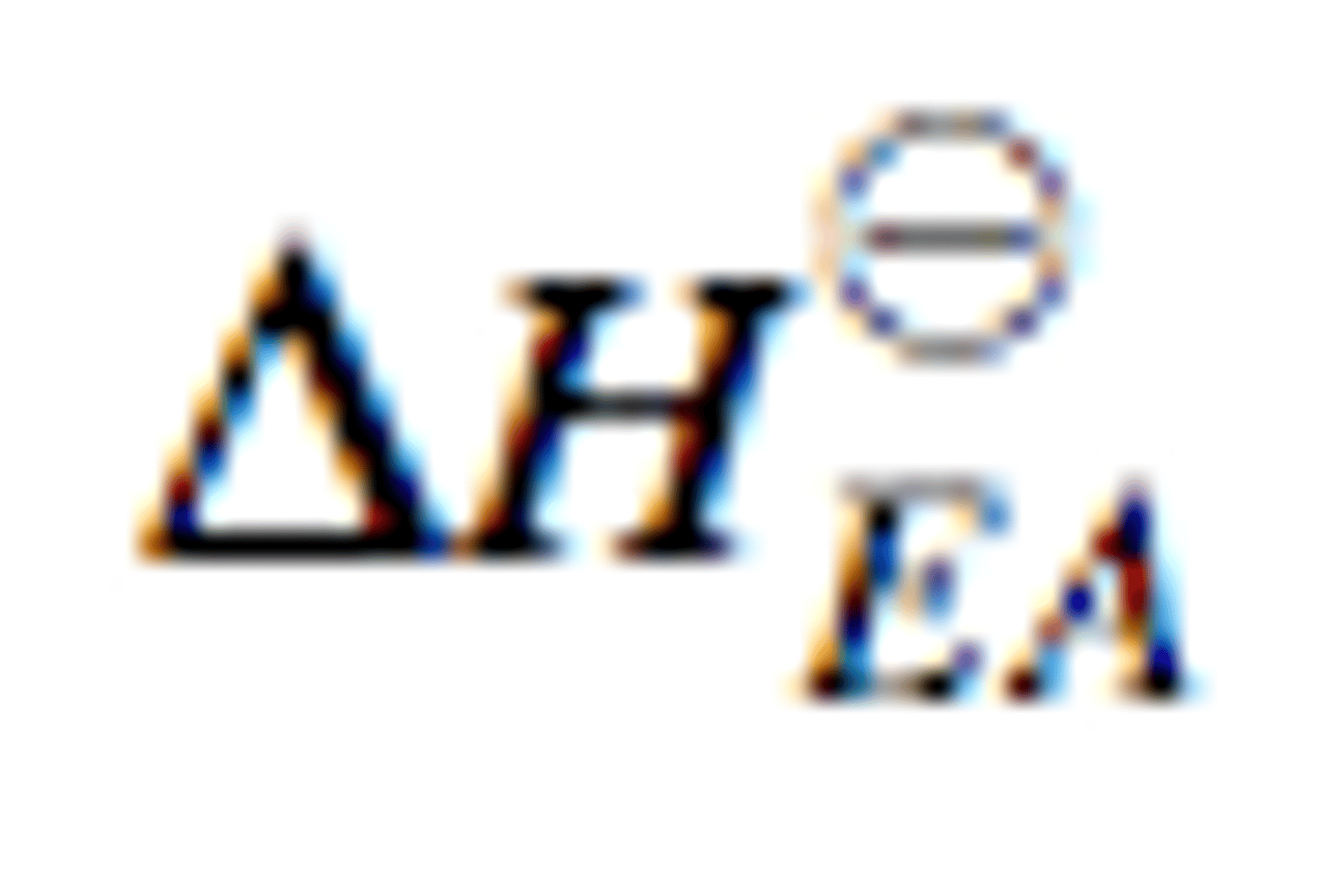
Are FIRST electron affinities exothermic or endothermic?
First electron affinities are exothermic the electron is being added is attracted by the positive nuclei
Why are second electron affinities endothermic?
- A second electron is being gained by a negative ion
- However there is now electron repulsion which repels the electron away.
- Energy must be put in to force the negatively charged electron onto the negative ion.
YOU MUST DO PRACTICE PAPER QUESTIONS
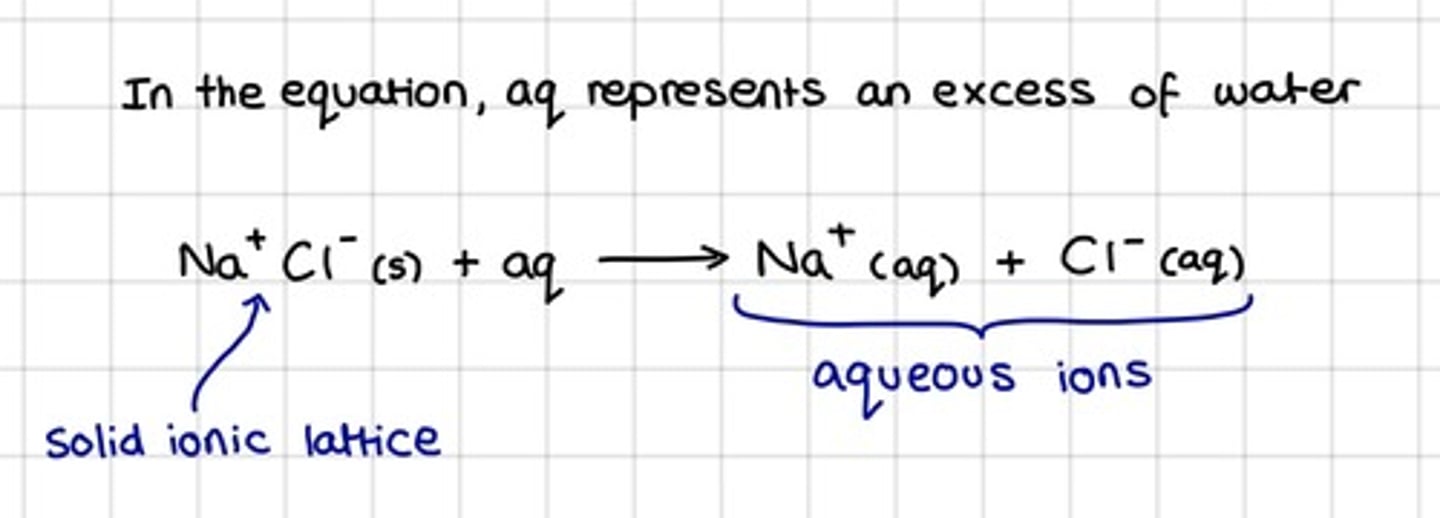
What happens when a salt dissolves in water?
Water molecules are able to break up the giant ionic lattice structure and overcome the strong electrostatic attractions between oppositely-charged ions
What is the enthalpy change of solution?
The enthalpy change that accompanies one mole of a solute dissolves in a solvent under standard conditions
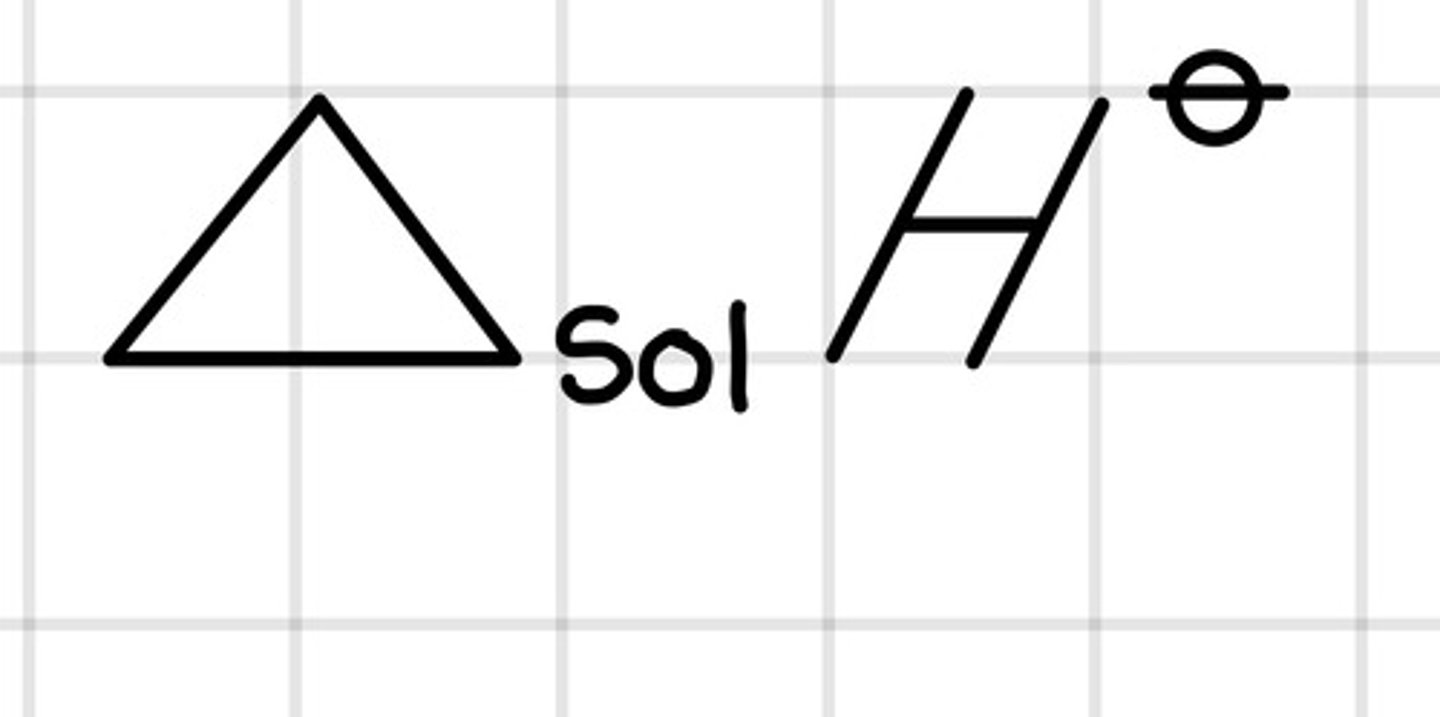
What is the standard enthalpy change of solution therefore associated with?
The dissolving process in a solvent
How do you represent the standard enthalpy change of solution in an equation?
∆solH⦵ = +4Jmol-1
What can the enthalpy change be in the standard enthalpy change of solution?
Endothermic or exothermic
In aqueous solution, which ion is attracted to the ∂- oxygen atom?
The positive cation
In aqueous solution, which ion is attracted to the ∂+ hydrogen atom?
The negative anion
How do you convert 5000 KJ/mol --> KJ provided you are given 5 moles?
5000/5 = 1000KJ Divide! Do not times!
What equation can be used to determine the ∆solH⦵?
q = mc∆t
When calculating the ∆solH⦵ using q = mc∆t, what do you need to be careful about?
Using the mass of the solution and water, not just the mass of one
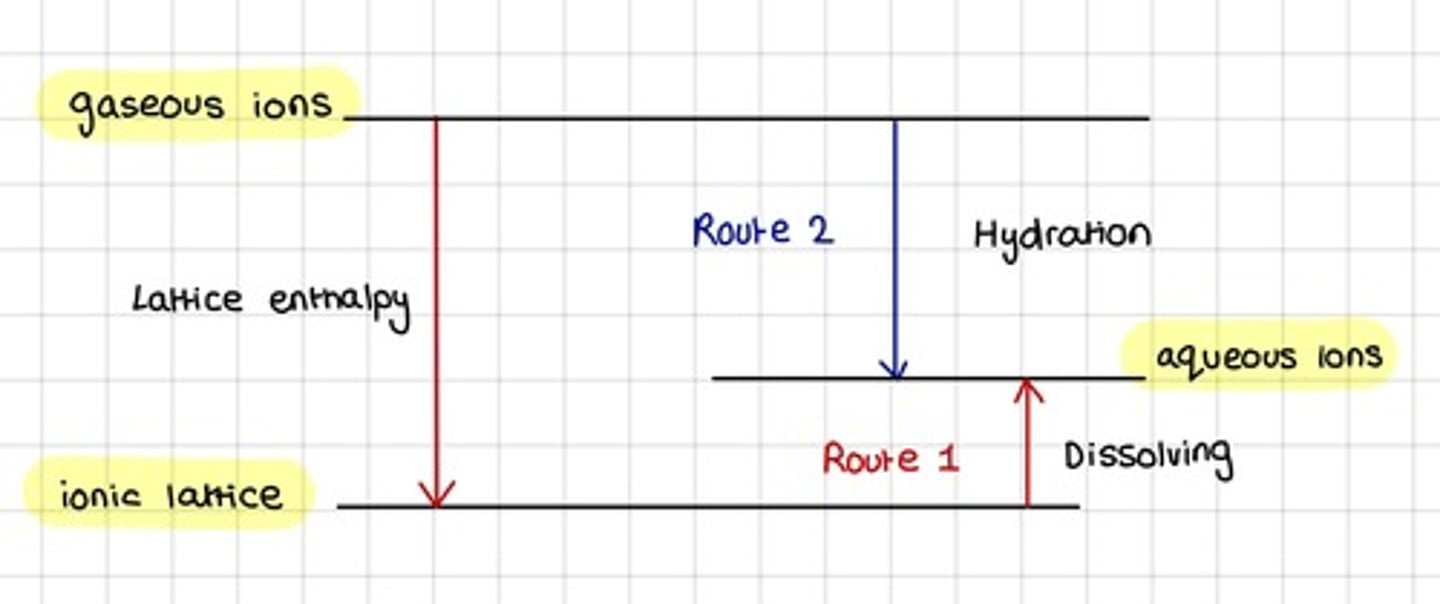
What two processes take place during the dissolving of a solid ionic compound?
- The ionic lattice breaks up
- Water molecules are attracted to and surround the ions
What energy change takes place during the breaking of the solid ionic lattice?
Opposite energy change of the lattice enthalpy from gaseous ions
What energy change takes place during the separate gaseous ions interacting with the polar water molecules to form hydrated aqueous ions?
Enthalpy change of hydration
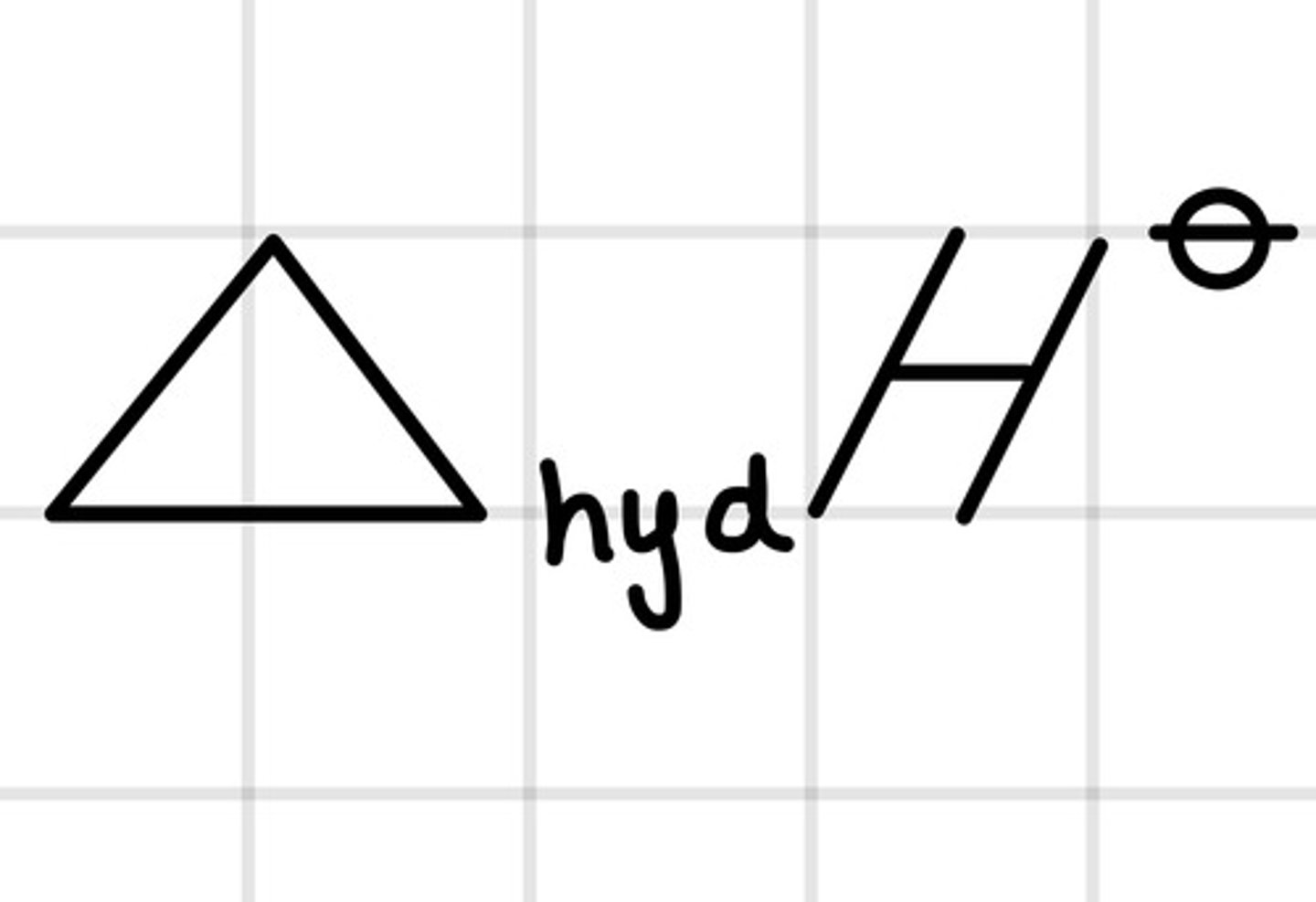
What is the exact definition for the standard enthalpy change of hydration?
Enthalpy change that takes place when the dissolving of gaseous ions forms exactly one mole of aqueous ions under standard conditions
Enthalpy change of hydration for magnesium ions
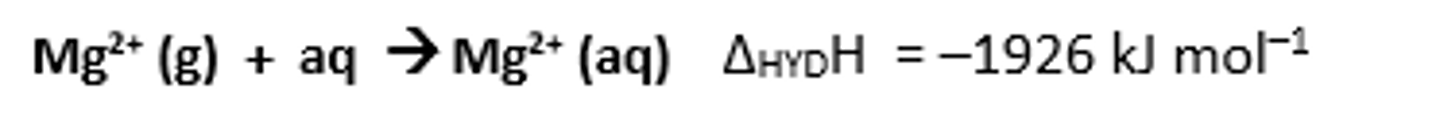
Energy cycle using standard enthalpy change of hydration and standard enthalpy change of solution
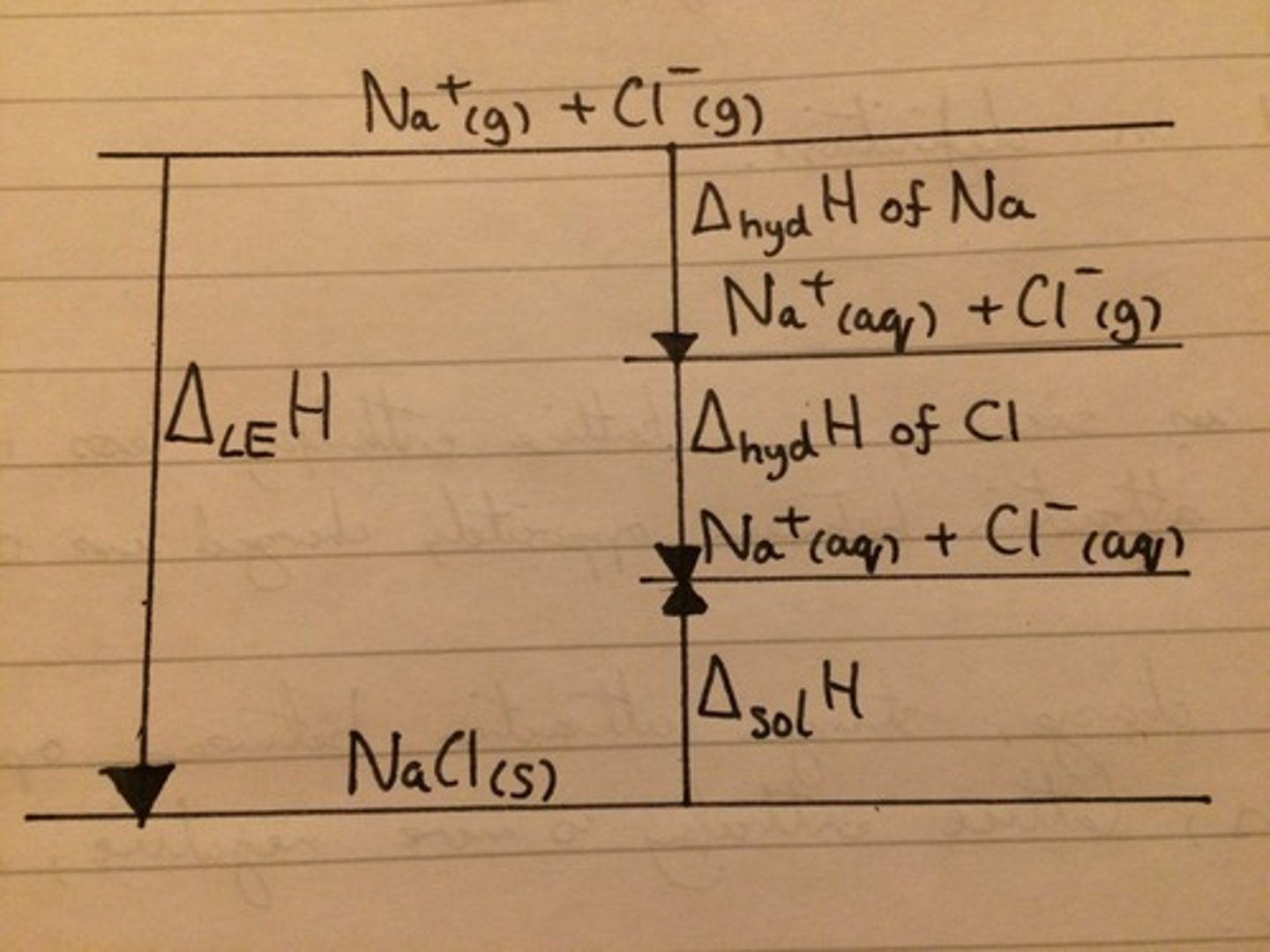
Energy cycle for sodium chloride:
Practice paper questions are needed desperately here as well
You, can, do this. A level exams will fear you!
What properties do ionic compounds have?
- High melting and boiling points
- Many compounds are soluble in polar solvents
- Conduct electricity when molten or dissolved in water
* This includes a wide range of ionic compounds
What are the factors affecting lattice enthalpy?
- Ionic size
- Ionic charge
What are the effects of ionic size increasing?
1) Ionic radii increases
2) Attraction between ions decrease
3) Lattice energy becomes less negative
4) Melting point decreases
What are the effects of ionic charge increasing?
1) Attraction between ions increases
2) Lattice energy becomes more negative
3) melting point increases
What are the two points to increasing attraction and therefore melting points?
- Increasing ionic charge
- Decreasing ionic size
What are the two points to decreasing attraction and therefore melting points?
- Decreasing ionic charge
- Increasing ionic size
Is lattice enthalpy a good indicator for melting points?
Yes
What are the factors affecting hydration?
- Ionic size
- Ionic charge
What does increasing ionic size do?
- Increases ionic radii
- Decreases the attraction between ion and water molecules
- Hydration energy becomes less negative
What does increasing ionic charge do?
- Increases the attraction between ions and water molecules
- Hydration energy becomes more negative
What are the two points for making the hydration energy more negative?
- Decrease ionic radii
- Increase the ionic change
- Increase the attraction between ions and water molecules
What are the two points for making hydration energy less negative?
- Increase ionic radii
- Decreases the ionic charge
- Decrease the attraction between ions and water molecules
How can you predict solubility of the dissolving of an ionic compound in water?
The sum of the energy change of hydration must be equal to or greater than the sum of the enthalpy change of lattice enthalpy
What is entropy?
Dispersal of energy within the chemicals making up the chemical system
What can entropy also known as in description?
- Randomness of particles
- Disorder
What can entropy be used to explain?
- Gas spreading through a room
- Heat from a fire spreading through a room
- Ice melting in a hot room
* Energy is being dispersed, becoming more spread out
Why is there always an entropy?
Always a natural tendency for energy to spread out rather than be concentrated in one place
What are the units for entropy?
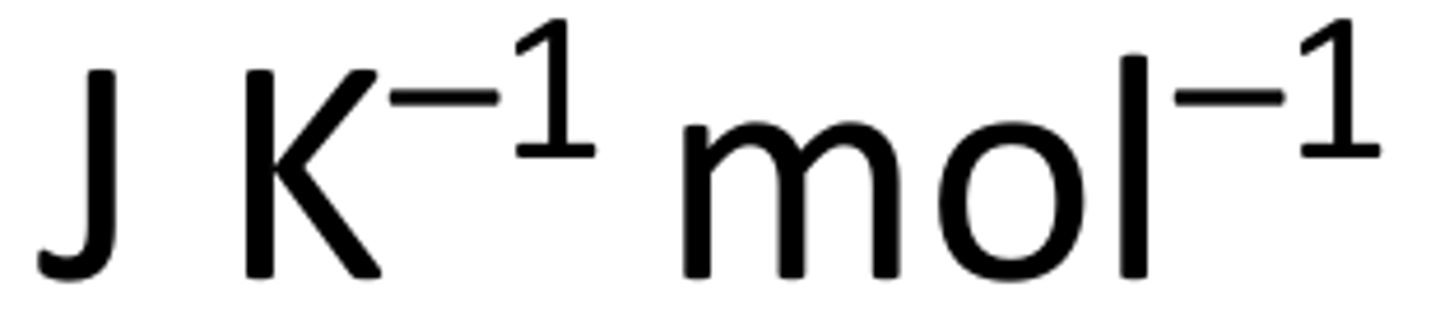
In general, what do different states dictate on entropy?
- Solids have the smallest entropies
- Liquids have the greater entropies
- Gases have the greatest entropies
* This rule only applies to individual substances
At absolute zero (0K) what energy do substances possess?
No energy
Above 0K, is the entropy for a substance always negative?
No, the entropy of a substance is always positive due to the dispersal of energy
What can systems with a higher entropy value be classed as?
Systems are classed as "more chaotic"
If a system becomes more dispersed and random, what will the entropy change be?
Positive +∆S
If a system becomes less dispersed and less random, what will the entropy change be?
Negative -∆S
How can you compare the entropy change between states of a specific substance?
Entropy increases during physical change for a more random arrangement of particles;
solid --> liquid --> gas
(--> indicates greater ∆S)
As a particular substance increases from a solid to liquid to gas, what three effects would occur?
- Melting points and boiling points would increase
- Disorder or randomness of particles would increase
- Energy would be more spread out and ∆S would be positive
What change in entropy would occur in reactions that produce gases?
- Production of a gas increases the disorder of particles
- Energy is more spread out, + ∆S
If a reaction occurred where there was a decrease in the number of gaseous moles, what would the entropy change be?
- Decrease number of gaseous moles and randomness of particles
- Energy is spread out less, -∆S
What is standard entropy?
Is the entropy of one mole of a substance under standard conditions
"S⦵"
What are the units for standard entropy?
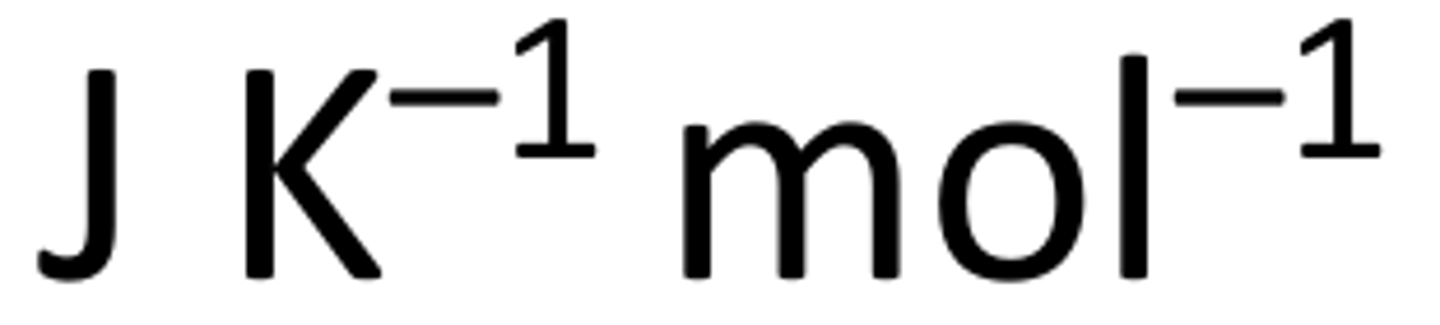
Are standard entropies always positive or negative?
Always positive
How do you calculate the the standard entropy change of a reaction?
Instead of " ˚ "
Use " ⦵ "
- Standard entropies of reactant should be given to you
- The only exception is if the standard entropy change of a reaction is given to you and you need to work out the standard entropy of one reactant

Do questions on entropy
Please. Also, you are amazing for revising this far
What is the main thermodynamic law of the feasibility for a reaction?
A reaction can happen only if the products have a lower overall energy than the reactants
What is feasibility?
Wether if a reaction is able to happen and if it it energetically feasible
What word can be used as synonyms for a reaction that is "energetically feasible"?
Spontaneous
What is free energy change? ∆G?
The overall change in energy during a chemical reaction
What two types of energy makes up the free energy change?
- Enthalpy change ∆H (heat transfer between chemical system and surroundings)
- Entropy change at the temperature of the reaction T∆S (dispersal of energy within the chemical system itself
State the Gibbs equation
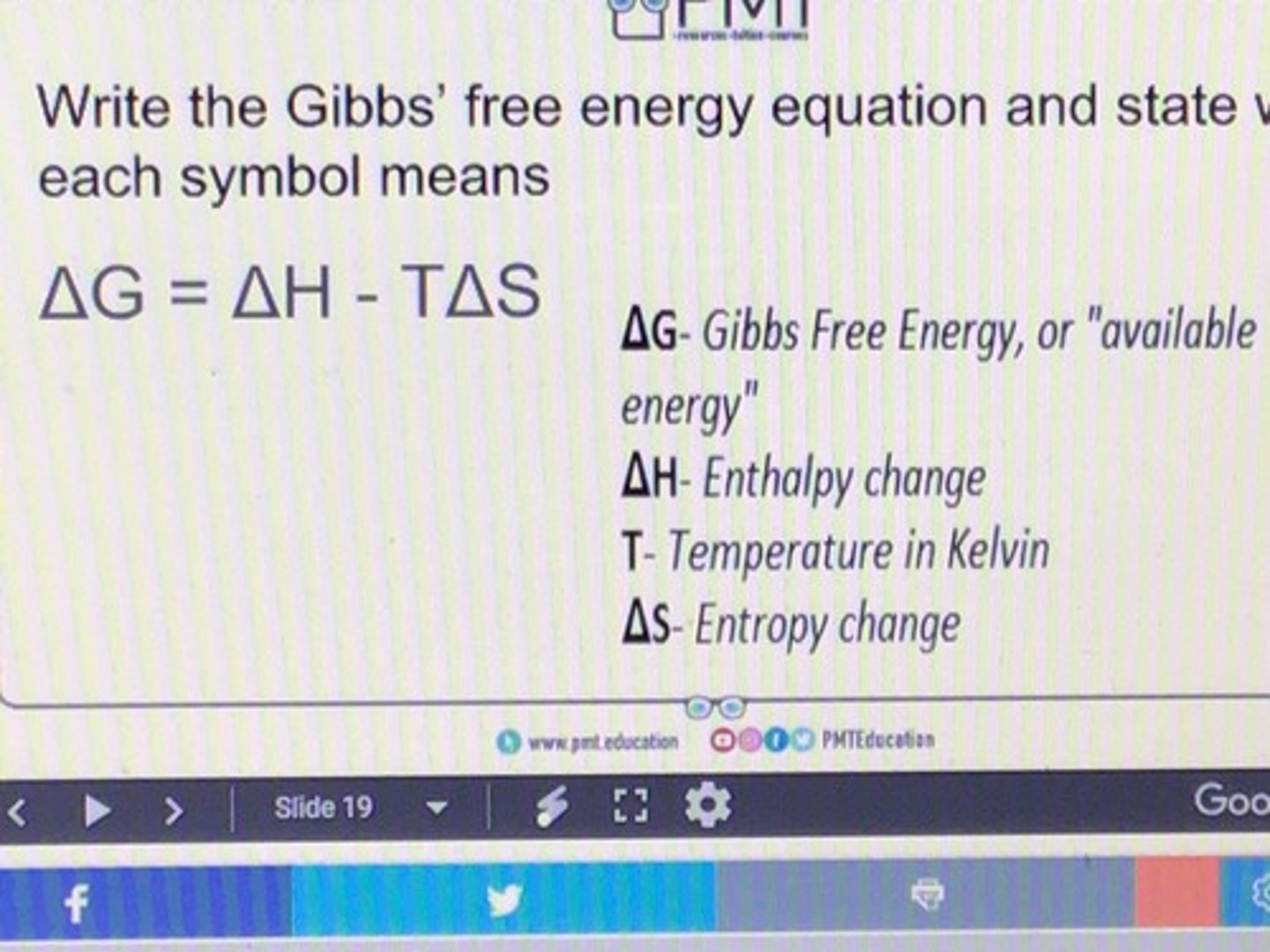
What two components does the feasibility of a reaction depend on?
Balance between ∆H and T∆S
What must occur for a reaction to be feasible?
Decrease in free energy;
* ∆G < 0
Which units must you change when doing your calculation of ∆G?
- The units of entropy (∆S) from J/K/mol --> KJ/K/mol
- This is done by dividing the entropy value by 1000
- Change ˚C to K
To know the minimum temperature for the feasibility of a reaction, what must you do?
- Rearrange for T
- Find T
- Place T greater than or equal to (T≥x) of a temperature
Why can endothermic reactions take place at room temperature?
As at 298K, ∆G for the endothermic reaction is energectically feasible
What is a limitation of the prediction of feasibility (∆G)?
While the free Gibbs equation will give you if a reaction is thermodynamically feasible, it will not provide;
- The activation energy for the reaction
- Therefore the kinetics of the reaction
- Therefore it will not provide the rate of reaction (reactions may seem to not be reacting but are at a ridiculously slow rate of reaction
What is one example of a feasible reaction but has a ridiculously slow rate of reaction?
- Diamond being turned to graphite at 4000 ˚C
- However this process takes millions if not billions of years
Practice. Paper. Questions.
I mean it!
What is OIL RIG?
- Oxidation Is Loss
- Reduction Is Gain
What two redox titrations will be need to be known in this A level exam?
- Potassium manganate(VII) KMnO4 (aq) under acidic conditions
- Sodium thiosulphate, NaS2O3
What are manganate titrations used for in analysis?
The analysis of reducing agents
What are two common examples of reducing agents?
- Iron (II) Fe2+(aq) ions
- Ethandioic acid (COOH)2 (aq) ions
Can the redox titrations of manganate (VII) be applied to any reducing agent?
No, only to reducing agents able to reduce MnO4- to Mn2+
Show the half equations and full equation for the redox titration of acidified manganate (VII) ions with iron (II) ions
(Top part of the picture)

What can KMnO4 be replaced with?
Potassium dichromate (k2Cr2O7)
Show the iodine thiosulphate half equations and equations
Picture

Why is the iodine-thiosulphate titrations needed?
- To determine the concentrations of aqueous iodide ions
- To determine the concentrations of oxidising agents
What other oxidising agents concentrations can be analysed using iodine thiosulphate titrations?
- Chlorate (I) ions ClO-
- Copper (II) ions, Cu2+
- Any other oxidising agents that can oxidise I- ions to I2
Why is the end point in a iodine thiosulphate titration difficult to analyse?
Fading of brown colour occurs gradually.
What is used to make the end point of a iodine thiosulphate more clear?
Starch indicator
With starch indicator, what is the colour of I2?
Blue black