Chapter 14 - Metabolic diversity of Microorganisms
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Which organisms use PMF
All mechanisms (except fermentation) use the proton motive force for the synthesis of ATP (oxidative and photo-phosphorylation)
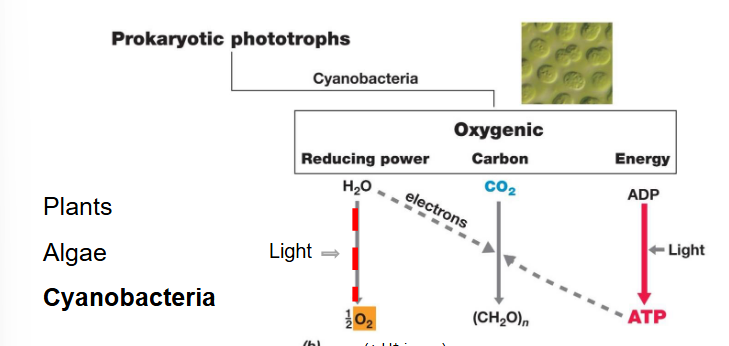
oxygenic vs anoxygenic photosynthesis
Oxygenic:
Electron donor: water
Produces: oxygen
Your average photosynthesis reaction
Uses both photosystems
Anoxygenic:
Electron donor: not water, other substances such as hydrogen sulfide, hydrogen, iron. (Chemotrophy)
Produces: not oxygen, other things such as elemental sulfur
uses only one photosystem (PSI)
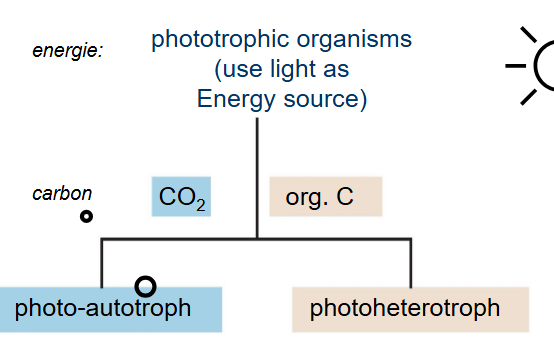
What cells do photosynthesis?
Phototrophs
AND a lot of bacteria
What colors are phototrophs
They come in all colors
People assume they are only green because of chlorophyll but this is not true
Does photosynthesis only produce oxygen?
There is also lots of anoxygenic photosynthesis
anoxygenic photosynthesis is a type of photosynthesis that does not produce oxygen. It’s carried out by certain bacteria (like purple sulfur bacteria) using molecules other than water (like hydrogen sulfide) as electron donors:
CO₂ + 2H₂S + light → CH₂O + H₂O + 2S
Problem with Oxygen in atmosphere
Creation of ozone upon uv light
Is photosynthesis well characterized?
Yes but there are many uncharacterized phototrophs in oceans
Two types of organisms doing photosynthesis
Oxygenic photosynthesis
Electron source: Water (H₂O) — they split water molecules by oxidation releasing electrons, releasing O₂
These electrons flow to a metabolic pathway that can fix CO2
Since CO2 fixation is expensive, light is used to turn ADP into ATP and this ATP also helps with CO2 fixation.
This ATP is ALSO used for water splitting
Anoxygenic photosynthesis
An electron donor like H2S is used and is oxidized to SO42- to release electrons that go towards carbon fixation
Here too, light is used to turn ADP into ATP, this energy is used for carbon fixation.
Here the ATP is not needed to help with water splitting because H2S is a much better electron donor.
Why is water used as an electron donor if it is a poor donor?
Is is available, very abundant
Name of scheme in oxygenic photosynthesis
Z-scheme
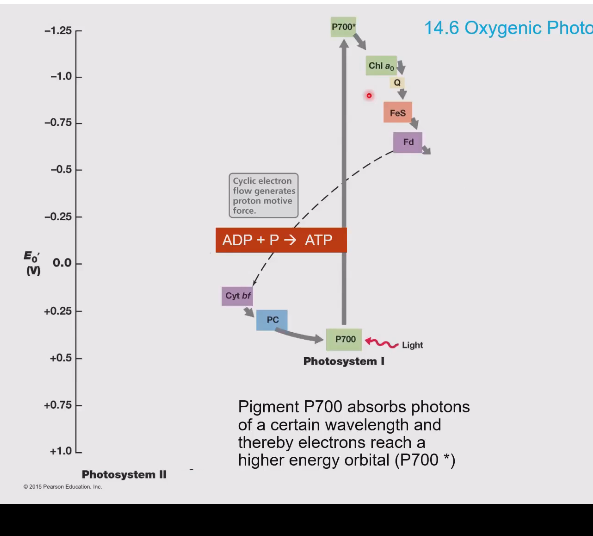
Z-scheme pathway (oxygenic photosynthesis)
Electrons are accepted in photosystem II, these electrons come from water splitting. These are accepted by P680 which is a really good electron acceptor.
The electron in the photosystem is now excited by light and goes up in energy to an excited state. This causes P680 to become a really good electron donor.
The electron goes through the electron transport chain (proton motive force!!) and slowly goes down in energy and moves into photosystem I. (P700)
In photosystem I it is excited again by light and eventually the electron moves into NAD(P)H
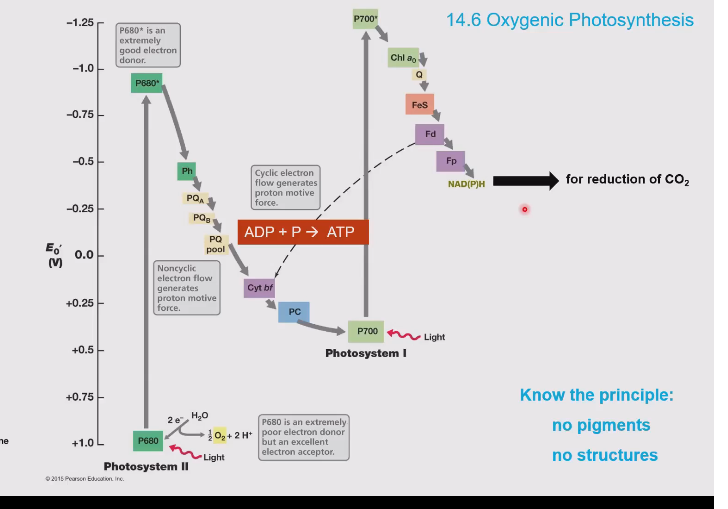
Photosystem I vs II
Photosystem I:
Produces NAD(P)H
Has P700 which absorbs light
Is second in the light dependent reactions
Photosystem II:
Splits water to generate:
Electrons (for the chain)
Protons (for ATP synthesis)
Oxygen (by product)
Has P680 to absorb light
First in the chain.
What happens if there are too many NADH molecules in oxygenic photosynthesis (so too many electrons)
Organisms will shut down photosystem II
Instead they create a proton motive force
So photosystem I still excites the electrons, then the electron falls down and releases energy which is used to create ATP.
This also means there is no oxygen formed because that only happens in photosystem II.
Heterocyst’s and photosynthesis
In the heterocyst’s photosystem II is switched off.
They only have photosystem I
Photosystem II produces oxygen and we don’t want that for the nitrogenase.
How are the electrons accepted in the photosystem?
By chlorophyll which love to receive oxygen
Electron flow in Anoxygenic photosynthesis
Pretty much only found in prokaryotes not eukaryotes
Contains bacteriochlorophyll a (instead of just chlorophyll)
Red or infrared light excites the pigment (P870)
The cyclic electron flow generates proton motive force.
Found in purple-sulfur bacteria
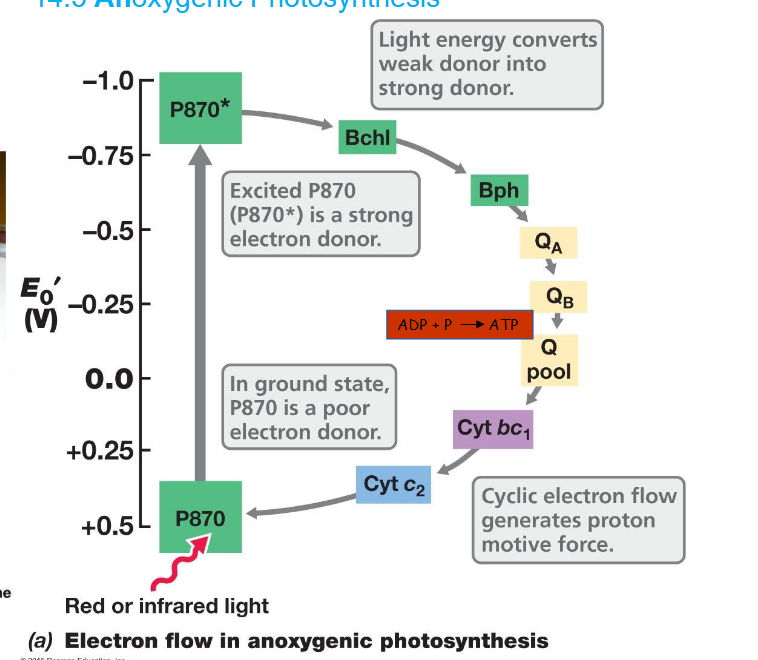
Reduction of NAD in anoxygenic photosynthesis
Even though electrons are cycling around, they cannot be used to reduce NAD (low reduction power) which is necessary for CO2 fixation in autotrophs.
Therefore reverse electron transport is used.
This allows reduction of NAD to NADH which is then used for CO2 fixation.
Through what process is CO2 reduced
The Calvin cycle
What is the most abundant enzyme on the planet?
RubisCO
Pretty inefficient → therefore you require a lot
¼ enzymes go wrong
The enzyme does not need to get better, no evolutionary pressure
Instead of making 3-P-glycerate it makes 2-P-glycolate
Calvin cycle general steps
Carboxylation 6CO2 molecules are turned into 12 C3 molecules with phosphate. This is done by rubisco.
ATP & NADPH is invested producing Ribulose 1,5 biphosphate
2 C3 molecules are lost so we are left with 10 C3 molecules
With ATP the 10 C3 molecules are turned into 6 C5 molecules.
chemoorganotrophy vs chemolithotrophy
Chemoorganotrophy:
Uses organic compounds are an electron donor
Uses carbon from substrate for cell material.
Does fermentation
Chemolithotrophy:
Uses an inorganic electron donor
Fix carbon from CO2
Hetero vs. Autotrophs
Hetero: carbon source is organic molecules
Auto: carbon source is carbon dioxide
The problem with chemolithotrophy
Chemolithotrophs use inorganic compounds as electron donors (energy source)
For that reason they are often autotrophic, and get their C from CO2.
The problem is that to do this you need another electron donor. (NADPH)
To be able to form NADPH from NADP, an electron donor is needed with a sufficiently negative potential.
For example H2 meets this requirement (above NADH in redox tower) but H2S does not (below NADH in redox tower)
Solutions for weak electron donors in chemolithotrophy
Coupling to ATP hydrolysis
Reversed electron transport
Electron bifurcation (mostly used in anaerobes)
Hydrogen oxidizing bacteria
organisms that combine hydrogen and oxygen → creates lots of energy
Have very fast growth
Many different species
Are chemolitho-autotrophs
Growth at low oxygen concentrations, because the hydrogenases are oxygen sensitive
Calvin-cycle for carbon fixation
Hydrogen is almost entirely derived from fermentative conversions
Electron transport scheme of hydrogen oxidizing bacteria
Hydrogen is used to generate proton motive force
Hydrogen is split up, protons are dumped out. Electrons are used to further the chain
Electrons go through the chain.
Proton motive force is created and can be used to create ATP
Additionally hydrogen is used to create NADH, this NADH is used to fix CO2
Oxidation of sulfur compounds
Sulfur can be an electron donor in this case
Sulfite (H2S) can be taken apart and be converted into sulfate (SO42-)
Can have sulfur globules for later use.
Many sulfur oxidizers are acid tolerant because they are producing sulfuric acid.
Sulfur reducing bacteria
They are often found in seawater where there is a lot of sulfate
Black sand on the beach is from sulfur reducing bacteria
Iron bacteria
Use iron as their electron donor.
Use Fe2+ and some oxygen to make Fe+3 , water and ATP
Very slow growth
This is because iron is a poor electron donor.
When Fe3+ reacts with water, it forms insoluble iron. This is a thermodynamic advantage.
Usually live in acidic environments
Iron bacteria without oxygen
Some can use nitrate as their electron acceptor
Electron transport chain with iron bacteria
Fe2+ is converted to Fe3+ this happens on the outside of the cell to prevent insoluble iron inside the cell.
Electrons are used to generate energy.
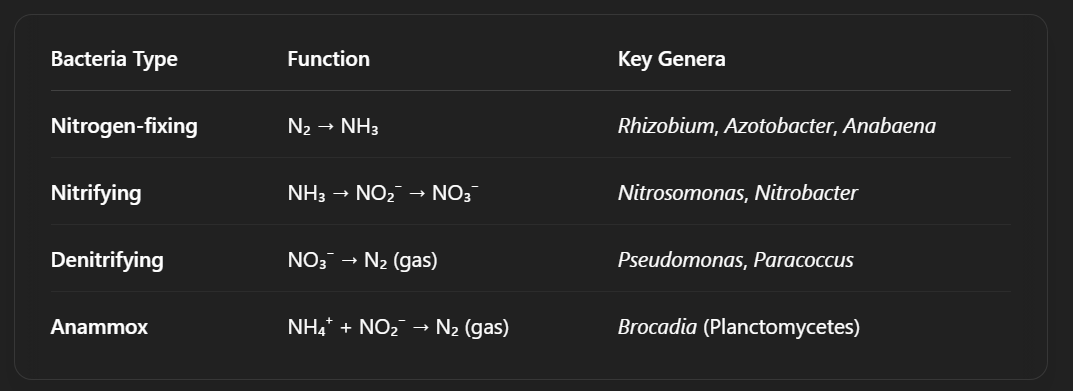
Nitrifying bacteria
Are widespread in soil, water and biofilters
They oxidize ammonia: NH3 → NO2-
And then oxidize nitrite: NO2- → NO3-
Then there are separate organisms that can take nitrite (which still has some electrons), put the electrons on oxygen to make nitrate and some ATP.
These two reactions often occur in two separate organisms because nitrite is a very toxic molecule. However recently it was discovered that there are organisms who can do both reactions. (Called Nitrospira)
Electron transport chain with nitrifying bacteria
Ammonia is oxidized
Electrons flow via cytochromes
The cytochromes are linked to oxygen, protons are pumped out
Protons go back in and produce ATP
Why do Nitrifying bacteria have a low growth rate and yield
They make a toxic intermediate (nitrite, NO2- )
The energy drop in the redox tower is not very high.
They are autotrophs so lots of energy goes to CO2 fixation.
Because NO2-/NH3 is below NADH in the redox tower, reverse electron transport is needed to build the NADH/NADPH pool which is needed for CO2 fixation.
How does the ANAMMOX process work in the absence of oxygen?
With nitrite.
Is done by ANAMMOX bacteria
Reaction: NH3 + NO2- → N2 + 2H2O + xATP
So NH3 is the electron donor and NO2- the electron acceptor.
Has a large vacuole called the anammoxosome, separate compartment for toxic intermediate.
Anammoxosome
Large vacuole found in bacteria that do not use oxygen.
Has a special membrane that protects cell against toxic intermediate H2N=NH2 (Hydrazine)
Hydrazine is used by rockets as fuel.
Special role of NO2- in creation of hydrazine
NO2- acts as electron acceptor for the oxidation of NH3
And as electron donor for the reduction of CO2
Two types of sulfate-reducing bacteria
Dissimilatory sulfate reduction: use of sulfate for respiration; production of lots of H2S
Assimilatory sulfate reduction: incorporation of sulfate for biosynthesis (synthesis of cysteine, methionine and other organic sulfur compounds)
Halorespiration
Respiration with all kinds of chloride compounds
Are often discovered in polluted sites
Other electron acceptors
Fe3+
Mn4+
AsO43-
Organisms that use CO2 as their electron acceptor
Very abundant but not very good.
Often occurs in places where there are not many other electron acceptors available.
Methanogens do this: Makes methane from CO2 (strictly anaerobic)
Acetogenesis: makes acetic acid (vinegar)
Assimilatory metabolism
Reductions to make cell material (amino acids)
Small amounts
No excretion
Costs energy
Dissimilatory metabolism
Reductions meant for obtaining energy (ATP)
Large amounts
Excretion of the reduced compounds.
What kind of bacteria that do something with nitrogen are out there?