Chemistry - 3.1.9: Rate Equations
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
The main factors that affect the rate of chemical reactions (5)
Temperature, concentration, pressure, surface area and catalysts
Rate of the reaction
The change in concentration (of any of the reactants or products) with unit time
When measuring the rate of reaction when the concentration of a single reactant changes, why should you add water? (2)
To make the volumes constant for all mixtures
So that volume of the reactant is proportional to concentration
How do you find the rate of reaction at a particular instant in time?
Draw a tangent and calculate the gradient
Rate expression
A mathematical expression showing how the rate of a chemical reaction at a particular temperature depends on the concentrations of chemical species involved
How is the rate equation determined?
Through experimental investigation
What does ∝ mean?
Proportional to
What is the symbol for rate constant?
k
Rate constant
The constant of proportionality in the rate expression
What is the rate expression in symbols?
rate = k[A]^m [B]^n, where A and B are the reactants of a chemical reaction
Is k always the same?
No - it differs for each reaction and varies with temperature
Order of reaction
The power to which the concentration of a reactant is raised in the rate equation
How do you find the total order of reaction?
Add the indices in the rate expression (m+n)
How do you describe the order of reaction for each species and overall for the rate equation?
It is m order with respect to A, n order with respect to B and (m+n) order overall.
Do all chemical species affect a chemical reaction?
No - some don't if they are zero order
Can catalysts appear in the rate equation?
Yes, as they affect the rate of a chemical reaction
How do you determine the units for the rate constant?
By cancelling out the units, where necessary
What does it mean if the reaction is zero order with respect to the species?
The rate is not affected by the concentration of the species
What does it mean if the reaction is first order with respect to the species?
The rate is directly proportional to the concentration of the species
What does it mean if the reaction is second order with respect to the species?
The rate is proportional to the square of the concentration of the species
2 ways you can find the order of a reaction
Rate-concentration graphs and the initial rate method
How do you find the order of a reaction by using rate-concentration graphs? (2)
1. From original graph of concentration against time, draw tangents at different concentrations, then calculate the gradient at each point to calculate the rate.
2. Plot a graph of rate against concentration.
3 possible rate-concentration graphs and their meanings
1. Graph is horizontal straight line - zero order (rate unaffected by concentration)
2. Graph is a sloping straight line through origin - first order (rate proportional to concentration)
3. Graph is not a straight line - cannot be found directly, might be second order
From a curved rate-concentration graph, how can you further determine if the order of reaction is 2?
Plot a graph of rate against concentration^2 - if straight line, second order
How do you find the order of a reaction by using the initial rate method? (3)
1. Carry out a series of experiments at a constant temperature with a different combination of initial concentrations of reactants, catalyst, etc - between any pair of experiments, the concentration of only one species should vary
2. Plot a concentration-time graph and calculate the initial rate by drawing a tangent at time = 0
3. Use this to create a table of initial rate and the initial concentrations of each reactant - these can then be compared to calculate the order with respect to each reactant
Arrhenius equation
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor (related to the number of collisions between reactant molecules), and e^(-EA/RT) is the fraction of collisions with enough energy to react
What is an alternate form of the Arrhenius equation, and how do you find it?
The logarithmic form of the equation, by taking ln of both sides
ln k = -Ea/RT + ln A
Why is it useful to use the logarithmic form of the Arrhenius equation?
The graph of ln(k) against 1/T will be a straight line with a gradient of -Ea/R
Logarithmic form of Arrhenius equation in the form y = mx + c
ln k = (-Ea/R)(1/T) + ln A
y = m x + c
Reaction mechanism
The separate steps that lead from reactants to products
Rate-determining step
The slowest step in the reaction mechanism that determines the rate of the overall reaction.
Alternate name for the rate-determining step
The rate-limiting step
Do steps that occur after the rate-determining step affect the rate of a chemical reaction?
No, as long as it is fast compared to the rate-determining step
Do steps that occur before the rate-determining step affect the rate of a chemical reaction?
Yes, as they affect the concentration of any intermediates in the involved in the rate-determining step
What should appear in the rate expression?
The reactants of the rate-determining step and any reactants in the reactions that come before it
How can you find the order of a reaction with respect to a given reactant from the steps in the reaction mechanism of a reaction?
By counting the number of times it appears in and before the rate-determining step
Units for the Arrhenius constant
s-1
When investigating the effect of the concentration of a reactant on the rate of reaction, why would you use a large excess of the other reactants? (2)
Their concentration is effectively constant, so they have no effect on the rate
When trying to determine the concentration of a reactant to investigate the order of that reactant in the reaction, what do you need to do to the sample between each experiment?
Stop the reaction by cooling it / adding a reagent to react with the other reactants
A+B→ C+D
X is a substance that can react with D to change colour from colourless to dark blue. All other substances are colourless.
Describe how to experimentally determine the order of reaction with respect to A. (7)
Measure volume of A and B, put A in measuring cylinder. Put B in a beaker
Measure volume of X and add to the beaker
Keep the temperature constant using a water bath
Mix and start stopwatch - measure time it takes for the solution to turn dark blue
Calculate the rate using 1/t
Repeat with different concentrations of A while keeping the concentration (and volume added) of B constant
Plot a graph of rate against concentration - use shape to determine the order
From a multistep reaction mechanism, how can you tell which step is the rate-determining step?
Pick the one with the same reactants as the rate equation - the species appear in the rate step in the same ratio as in the rate equation
Why dos increasing the temperature increase the rate of a reaction more than doubling the concentration of a reactant? (3)
Reaction can only occur when molecules have more energy than Ea
Increasing temperature causes many more molecules to have energy >/= Ea
Doubling concentration only doubles number of concentration
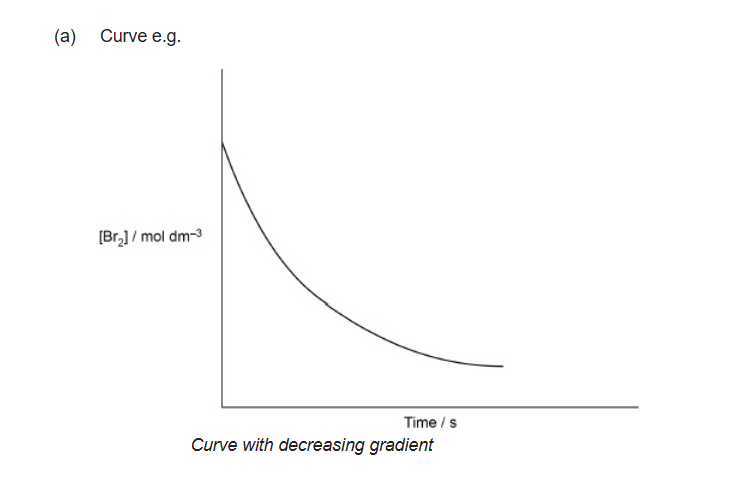
CH3COCH3 + Br2 + OH– ⟶ CH3COCH2Br + Br– + H2O
Rate = k [CH3COCH3] [OH–]
Sketch a graph to show how the concentration of bromine changes during this reaction at constant temperature.
How can you tell the order of a reaction from a table with T, 1/T, k and ln k?
From the units of k