Chapter 18: The Chemistry Of Aryl Halides, Vinylic Halides, and Phenols
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
18.1 Lack Of Reactivity Of Vinylic And Aryl Halides Under SN2 Conditions
What is a contrast between vinylic or aryl halides and alkyl halides?
Simple vinylic and aryl halides are inert under SN2 conditions
Example:
When ethyl bromide is allowed to react with Na+ EtO− in EtOH solvent at 55 °C, the following SN2 reaction proceeds to completion in about an hour:

Yet when vinyl bromide or bromobenzene is subjected to the same conditions, nothing happens!
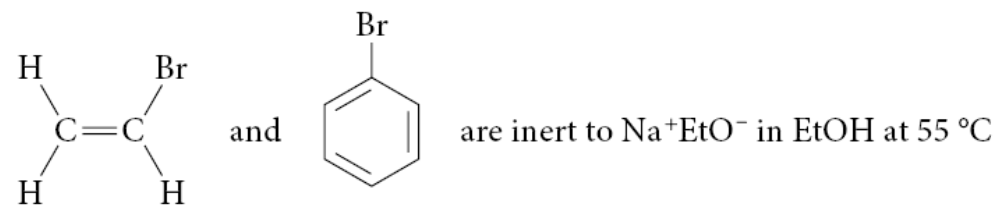
Why can’t vinylic halides undergo SN2 reactions?
Vinylic halides do not undergo SN2 reactions because the substitution would require rehybridization from sp² to sp, which is highly unfavorable. Unlike alkyl halides, where the carbon shifts from sp³ to sp² in the transition state, vinylic halides already have an sp²-hybridized carbon involved in a double bond. Forming an sp-hybridized transition state is too unstable, preventing the SN2 mechanism
Vinylic halides are unreactive in SN2 reactions not only due to unfavorable rehybridization but also because of steric hindrance. The C=C–halogen bond angle changes from ~120° to 90° in the transition state, bringing the nucleophile and leaving group close to the adjacent alkene substituents. This causes strong van der Waals repulsions, especially when the adjacent groups are larger than hydrogen, raising the transition state energy and further slowing the reaction
Summary: Both hybridization and van der Waals repulsions (steric effects) within the transition state retard the SN2 reactions of vinylic halides to such an extent that they do not occur.
18.2 Elimination Reactions Of Vinylic Halides
Although SN2 reactions of vinylic halides are unknown, what can occur with vinylic halides?
Base-promoted beta-elimination reactions of vinylic halides do occur and can be useful in the synthesis of alkynes
Example:
Note: the second example shows two successive eliminations taking place. The first gives a vinylic halide, and the second gives the alkyne.
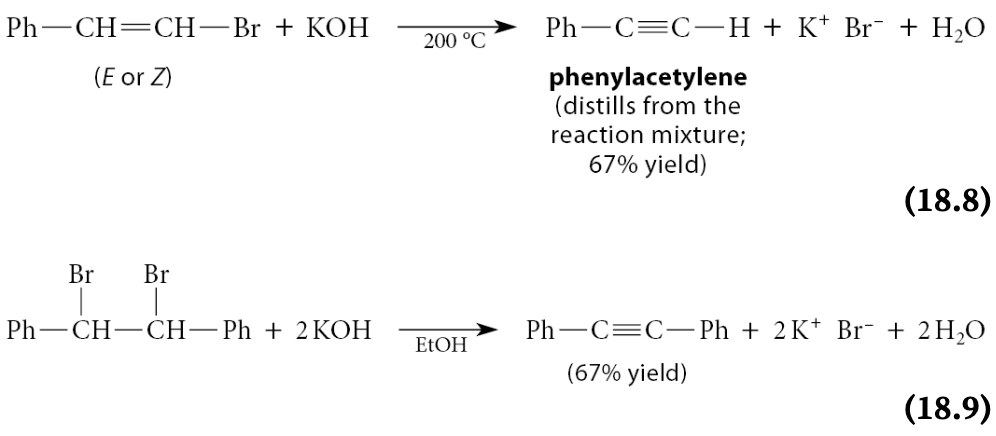
What do many vinylic eliminations require?
Harsh conditions (heat or very strong bases), and some of the more useful examples of this reaction involve elimination of beta-hydrogens with enhanced acidity
18.3 Lack Of Reactivity Of Vinylic And Aryl Halides Under SN1 Conditions
What can happen to tertiary and some secondary alkyl halides?
They can undergo nucleophilic substitution and elimination reactions by the SN1 and E1 mechanisms
Example:
Tert-butyl bromide undergoes a rapid solvolysis in ethanol to give both substitution and elimination products.
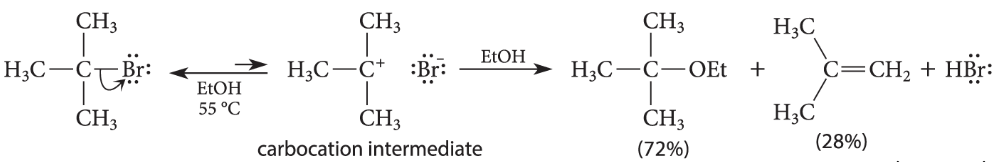
Vinylic and aryl halides are virtually inert to the conditions that promote SN1 or E1 reactions of alkyl halides. Although uncommon, certain vinylic halides can be forced to react by the SN1-E1 mechanism. How?
Certain vinylic halides can be forced to react by the SN1–E1 mechanism under extreme conditions, but such reactions are relatively uncommon
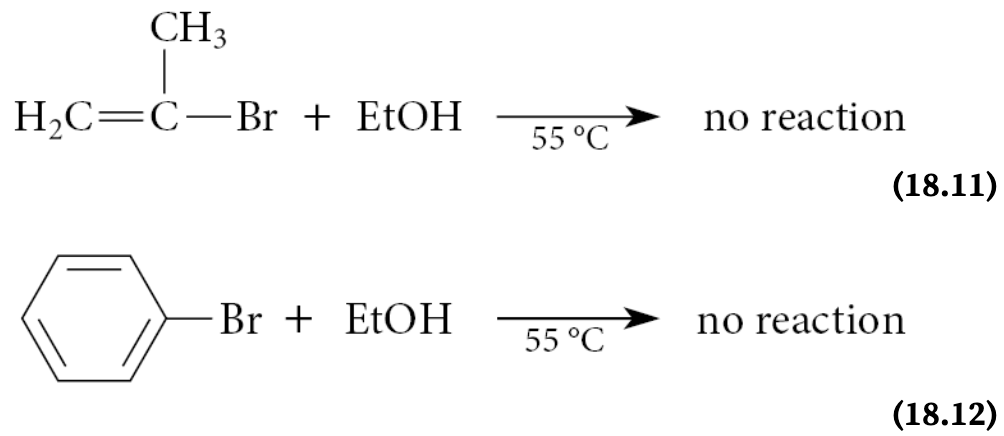
What happens if a vinylic halide undergoes an SN1 reaction?
It must ionize to form a vinylic cation
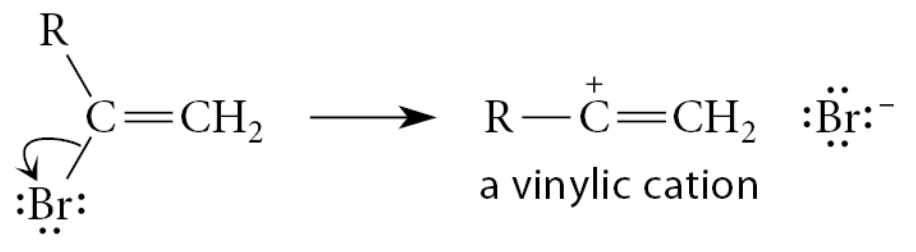
What are the reasons for why vinylic halides do not undergo SN1 reactions?
One reason that vinylic halides do not undergo the SN1 reaction is the instability of the vinylic cations that would necessarily be involved as reactive intermediates.
A second reason vinylic halides do not undergo SN1 reactions is because their carbon–halogen bonds are stronger than those in alkyl halides. Since vinylic carbons are sp²-hybridized, their bonds have more s character, making them stronger and harder to break. This higher bond strength increases the energy required for ionization, slowing the reaction.
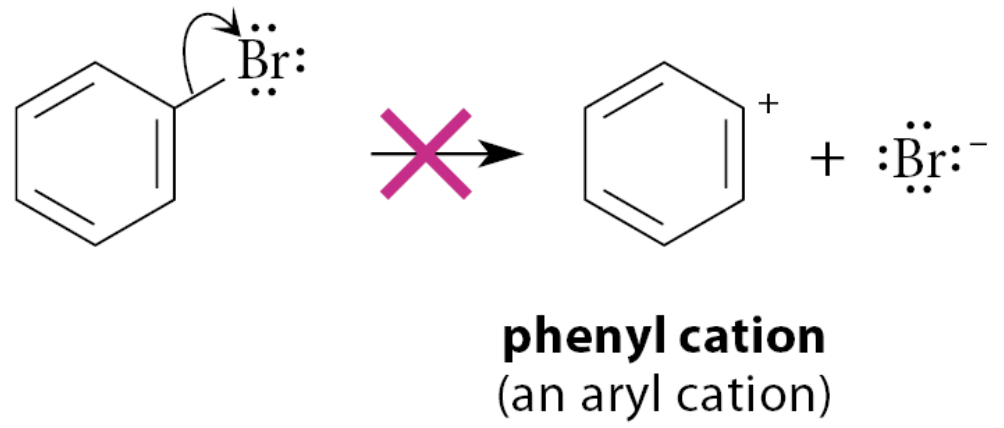
What does the SN1 reaction of an aryl halide involve?
It would involve an aryl cation as a reactive intermediate
Aryl cation: a carbocation in which the electron-deficient carbon is part of an aromatic ring
SN1 reactions of aryl halides do not occur because they would require the formation of carbocation intermediates—aryl cations—with very high energy.
18.4 Nucleophilic Aromatic Substitution Reactions Of Aryl Halides
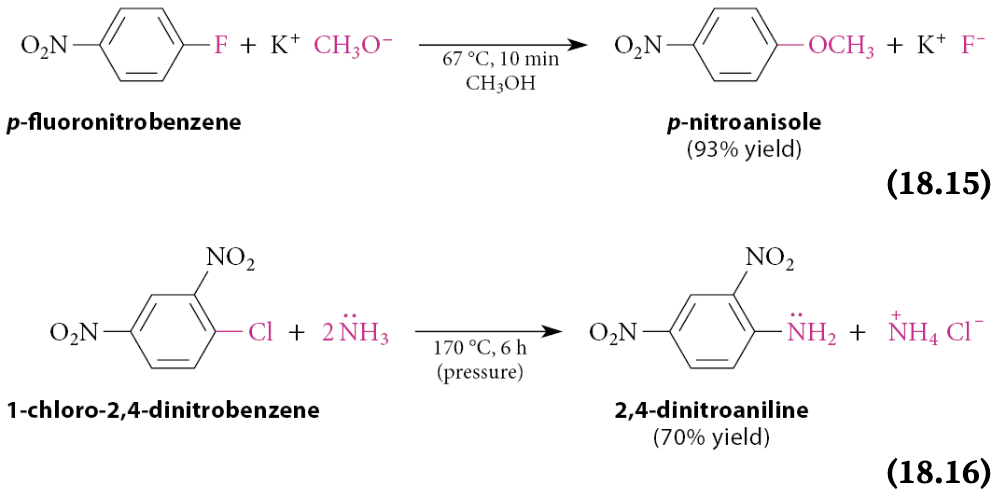
Although aryl halides do not undergo nucleophilic substitution reactions by SN1 and SN2 mechanisms, what does one or more nitro groups ortho or para to the halogen cause?
Aryl halides that have one or more nitro groups ortho or para to the halogen undergo nucleophilic substitution reactions by a different mechanism under relatively mild conditions
These reactions are examples of nucleophilic aromatic substitution
Like SN2 reactions, what do nucleophilic aromatic substitution reactions involve?
It involves nucleophiles and leaving groups and they also obey second-order rate laws
What does nucleophilic aromatic substitution reactions not involve?
A concerted opposite-side substitution
In nucleophilic aromatic substitution, when is the reaction faster?
The reaction is faster when there are more nitro groups ortho and para to the halogen leaving group:
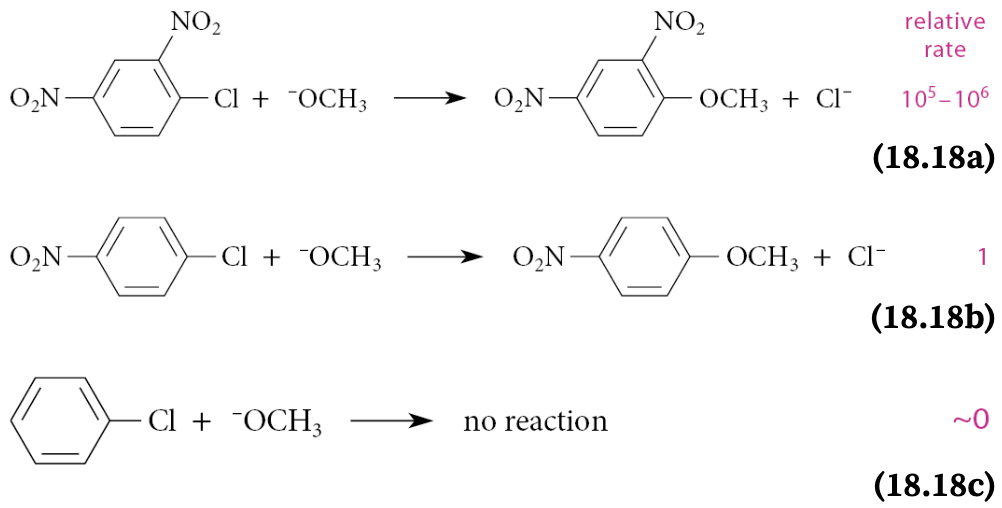
What effect do halogens have in nucleophilic aromatic substitution?
In nucleophilic aromatic substitution reactions, aryl fluorides are most reactive
In SN2 and SN1 reactions of alkyl halides, the reactivity order is exactly the reverse: alkyl fluorides are the least reactive alkyl halides
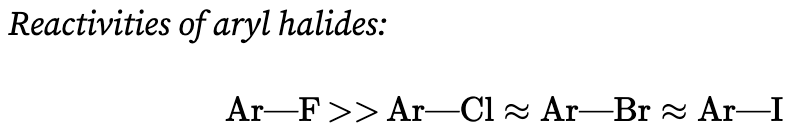
What is the reaction mechanism for nucleophilic aromatic substitution?
The nucleophile reacts at the halide-bearing carbon above or below the plane of the aromatic ring to yield a resonance-stabilized anion called a Meisenheimer complex
The negative charge is delocalized throughout the π-electron system of the ring
Formation of this anion is the rate-limiting step in many nucleophilic aromatic substitution reactions.
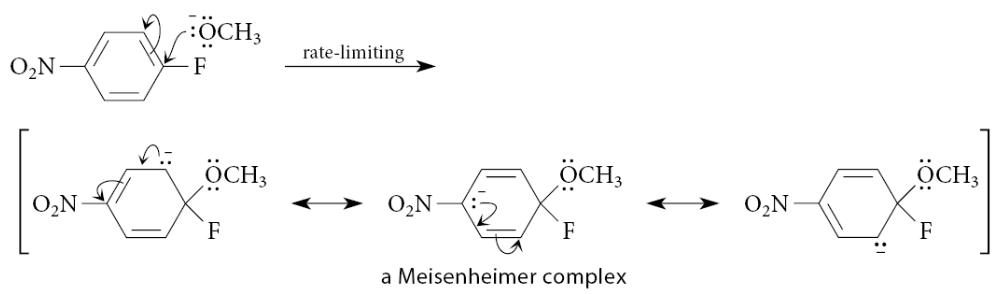
What can happen to the negative charge in the complex?
It can be delocalized into the nitro group?
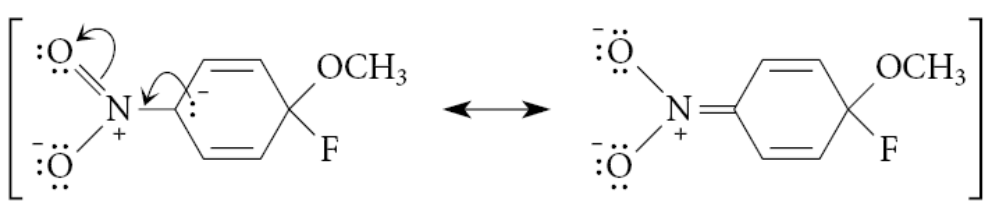
What happens to the Meisenheimer complex?
It breaks down to products by loss of the halide ion
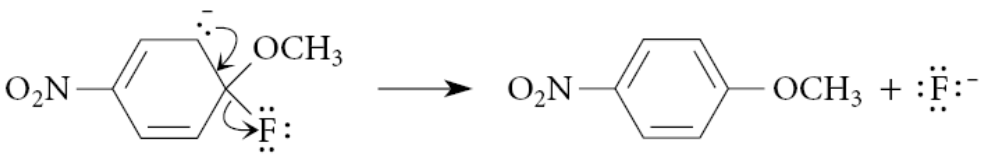
What accelerates nucleophilic aromatic substitution?
Ortho and para nitro groups accelerate the reaction because the rate-limiting transition state resembles the Meisenheimer complex, and ortho and para nitro groups (but not meta nitro groups) stabilize this complex by resonance
What stabilizes the negative charge?
Fluorine also stabilizes the negative charge by its electron-withdrawing polar effect, which is greater than the polar effect of the other halogens
What is not important in determining the reaction rate?
Because the loss of halide is not rate-limiting, the basicity of the halide—or, equivalently, the strength of the carbon–halogen bond—is not important in determining the reaction rate
How does nucleophilic aromatic substitution differ from SN2?
Nucleophilic aromatic substitution differs from SN2 in three key ways.
First, it involves a stable intermediate called the Meisenheimer complex, unlike SN2, which has no intermediate.
Second, it proceeds via frontside substitution without inversion of configuration, while SN2 requires backside attack with inversion.
Third, aryl fluorides react fastest in nucleophilic aromatic substitution, whereas alkyl fluorides react slowest in SN2 reactions.
18.5 Introduction To Transition-Metal-Catalyzed Reactions
Aryl or vinylic halides do not undergo SN1 or SN2 reactions. However, reactions that look very much like nucleophilic substitutions can be carried out with what?
Reactions that look very much like nucleophilic substitutions can be carried out using certain transition-metal catalysts
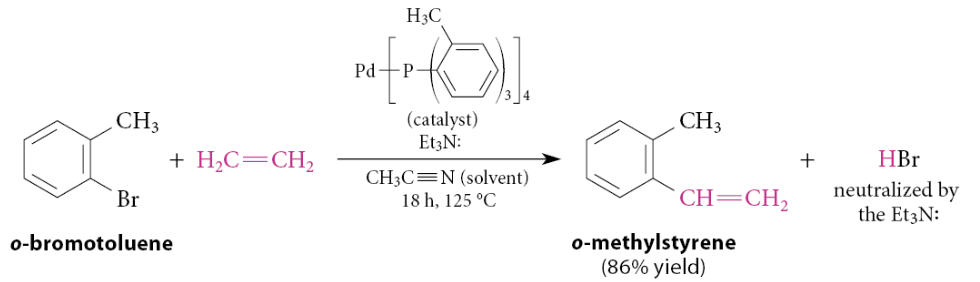
Example:
This is the Heck Reaction
Notice the formation of the carbon–carbon bond and the release of bromide as HBr. It looks as if the conjugate-base anion of ethylene displaces bromide ion from the aromatic ring. This reaction occurs by a very different mechanism and does not happen without the palladium catalyst.
Where can transition metals be found?
In the “d block” or “B” groups of the periodic table (groups 3-12 in the IUPAC numbering)
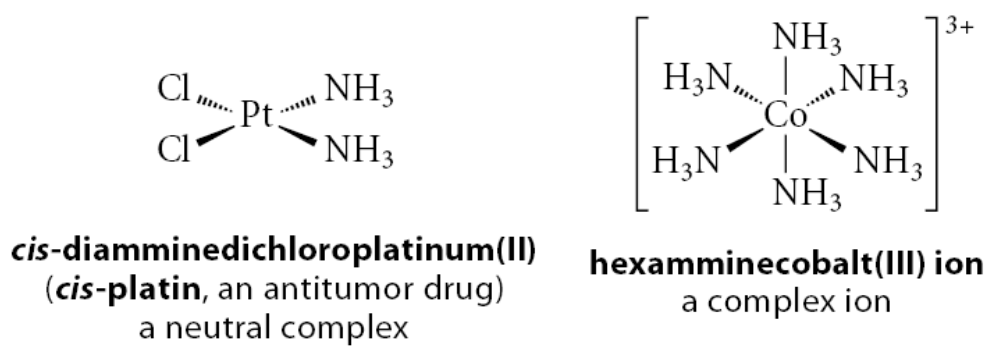
What is a ligand?
Central to transition-metal chemistry are a wide variety of compounds containing transition metals surrounded by several groups, called ligands . Such compounds are called coordination compounds or transition-metal complexes . These can be neutral molecules, as in the first of the following examples, or complex ions, as in the second example:
What are all ligands?
Lewis bases, they interact with transition metals by donating electron pairs
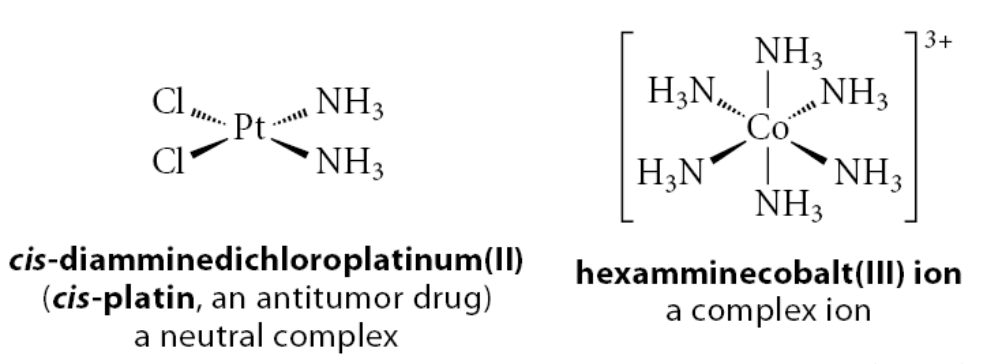
What are L-type ligands?
If a ligand becomes a neutral molecule when it dissociates from the metal with its bonding electron pair
Example:
Any one of the NH3 ligands in the complexes shown is an L-type ligand because if we remove it with its bonding electron pair, we get :NH3, the neutral molecule ammonia
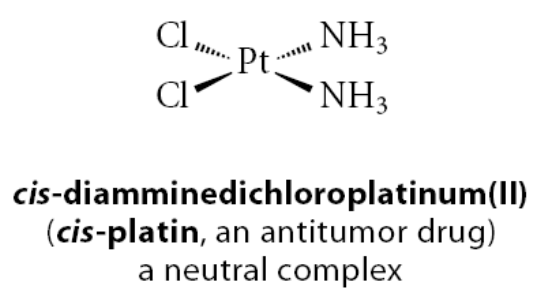
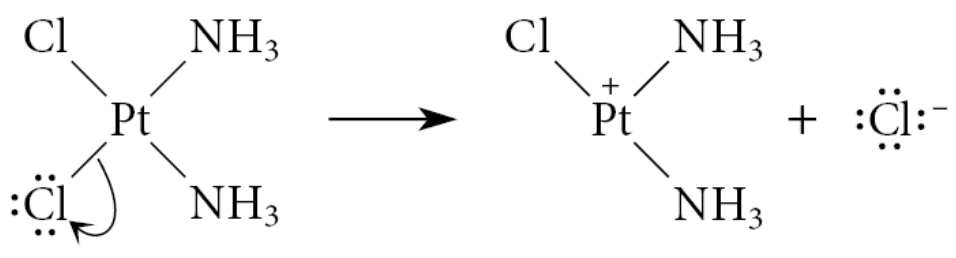
What is an X-type ligand?
If a ligand becomes a negative ion when it dissociates from the metal with its bonding electron pair
Example:
The Cl in cis-platin is an X-type ligand, because removing it with its bonding pair of electrons gives the chloride ion, Cl−
From a formal-charge perspective, the bonding electrons belong to what in L-type ligands?
The bonding electrons on L-type ligands are considered to “belong” completely to the ligand
What is a dative bond?
Leaving the bonding electron pair on the ligand and depicting the ligand–metal bond as an arrow from these electrons to the metal
Used for L-type ligans
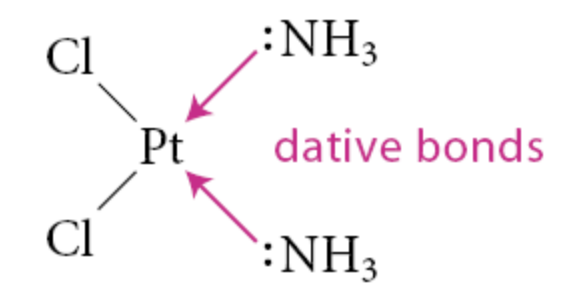
How are electrons in bonds assigned in X-type ligands?
Electrons in the bonds to X-type ligands are assigned in the same way that we assign electrons in main-group chemistry: one electron is assigned to the ligand and one to the metal
This means that if we remove an X-type ligand, it takes on an additional negative charge and the metal takes on a compensating positive charge:
What are allyl and cyclopentadienyl (Cp) classified as?
Allyl and cyclopentadienyl (Cp) are classified as both L-type and X-type ligands
One X-type bond accounts for the fact that Cp takes on one negative charge when removed with a bonding pair from the metal, and that the four remaining π electrons (that is, two double bonds) take part in two L-type bonds. In other words, we can think of a metal–Cp complex in the following way (M=metal):
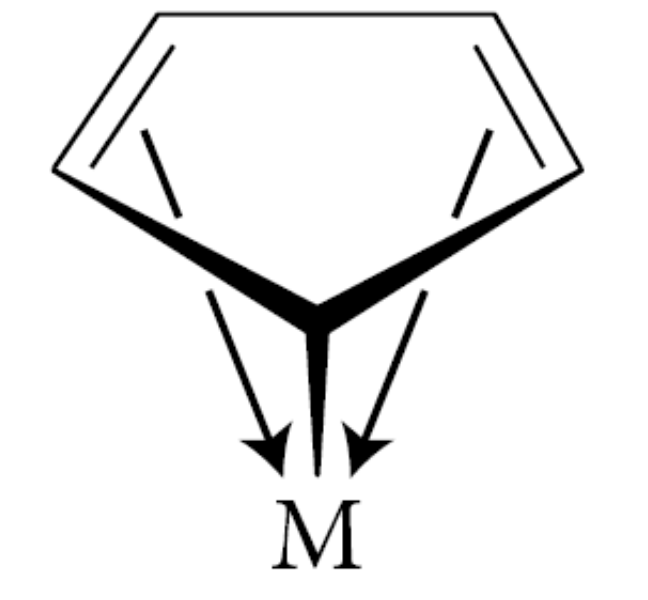
What is oxidation state?
The charge the metal would have if all X-type ligands were dissociated with their bonding electron pairs
Do L-type ligands contribute to the oxidation state?
No
What is dn notation?
The number of nonbonding valence electrons on the metal is the number n
What is the formula for dn?
n = valence electrons in neutral transition metal - oxidation state of transition metal
What is the formula for electron count?
electron count = n + 2(number of all ligands)
What happens in ligand dissociation?
A ligand simply departs from the metal with its pair of electrons, leaving a vacant site (orbital) on the metal
This process does not change the oxidation state of the metal, but it does change the electron count
Its reverse is ligand dissociation
What is ligand substitution (transmetallation)?
A ligand substitution can occur by the dissociation of one ligand and the association of another
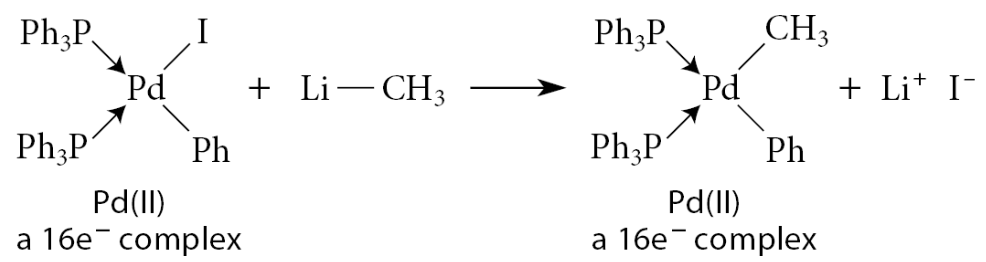
What is oxidative addition?
A metal M reacts with a compound X—Y to form a compound X—M—Y; the metal “inserts” into the X—Y bond
The metal inserts into a chemical bond within a compound not initially associated with the metal.
Example:
Both of the new bonds are X-type bonds. As a result of this reaction, both the electron count and the oxidation number of the metal increase by 2 units.
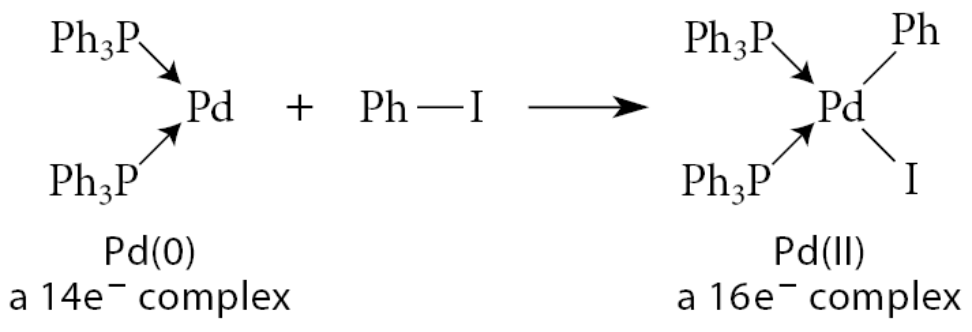
What is reductive elimination?
Conceptually the reverse of oxidative addition
Two ligands bond to each other and their bonds to the metal are broken; X—M—Y → X—Y+M
An example of this process is the formation of a carbon–carbon bond between two ligands within a Ni complex:
Because two X-type ligands are lost, the metal is reduced, and its electron count is decreased.
In this particular example, the alkene stereochemistry is retained.
Reductive elimination in general occurs with retention of stereochemistry
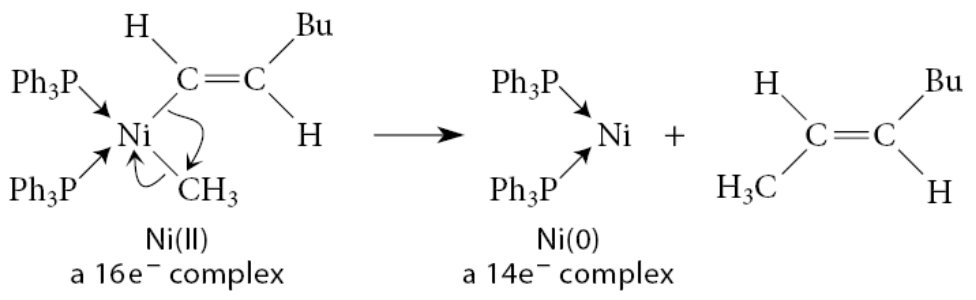
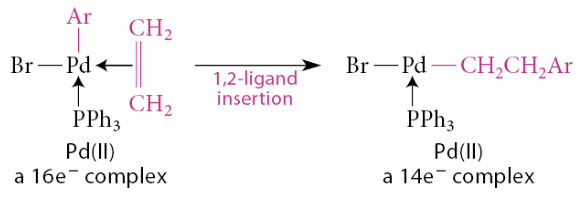
What is ligand insertion
A ligand inserts into a metal–ligand bond—that is, L—M—R → M—L—R
A ligand L inserts into the bond between the metal and a different ligand R, and the inserting ligand gains a bond
The metal inserts into a chemical bond within a compound not initially associated with the metal
Ligand insertion is a syn addition
New bonds must be formed at the same face of the pi bond
Two types of ligand insertion:
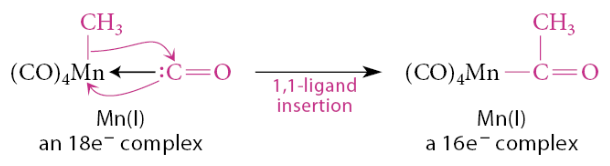
In a 1,1-insertion, the new bond is formed at the same atom that was bound to the metal
In this reaction, the methyl group migrates, with its bonding electrons, to the carbon of the carbonyl ligand, which in turn forms an X-type bond to the metal
In a 1,2-insertion, the migrating group moves to an atom adjacent to the one bound to the metal
The electron count is reduced by two
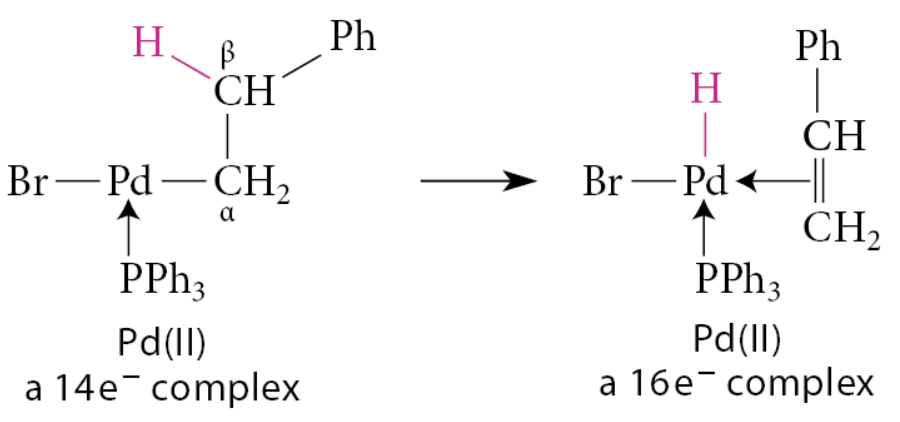
What is β-elimination?
A group β to the metal migrates with its bonding electron pair to the metal
Conceptually the reverse of ligand insertion
Electron count is increased by 2 units
Must occur as a syn elimination
Requires empty orbital on transition metal
Many β-elimination reactions involve a hydride migration
18.6 Examples Of Transition-Metal-Catalyzed Reactions
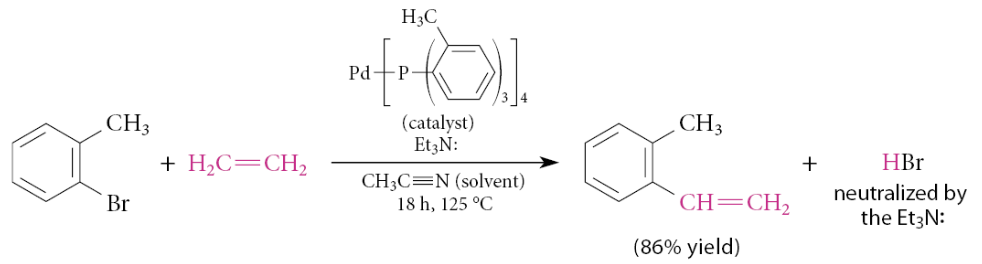
What is the heck reaction?
An alkene is coupled to an aryl bromide or aryl iodide under the influence of a Pd(0) catalyst
What is thought to be the catalytically active species?
PdL2, formed by 2 ligand dissocations
What happens to PdL2?
It enters the catalytic cycle
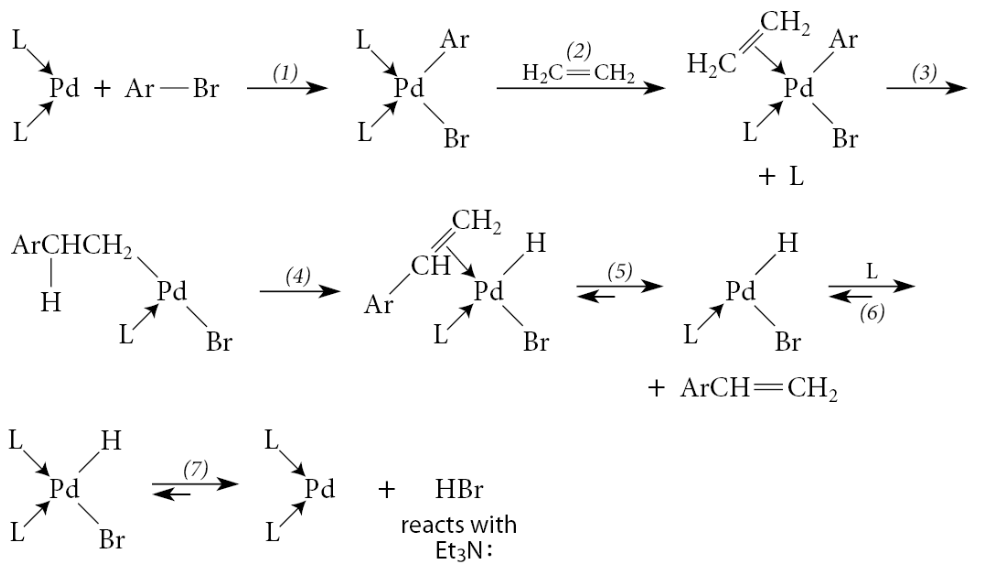
What 2 important aspects of the Heck reaction does the following example depict:
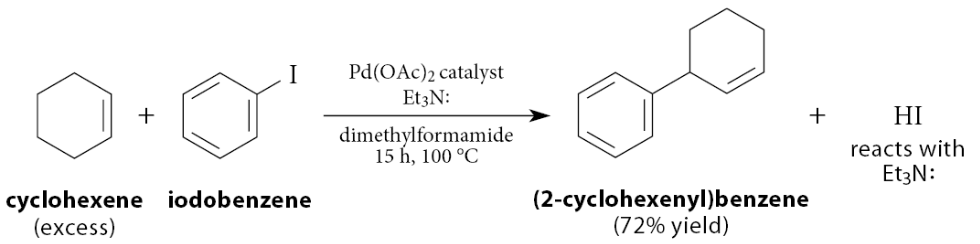
The catalyst Pd(0) is generated from a Pd(II) compound, Pd(OAc)2, which is used because it is a convenient and easily handled Pd derivative
The alkene double bond in the product is not at the site of coupling, but rather one carbon removed
Why does is the alkene double bond in the product not at the site of coupling?
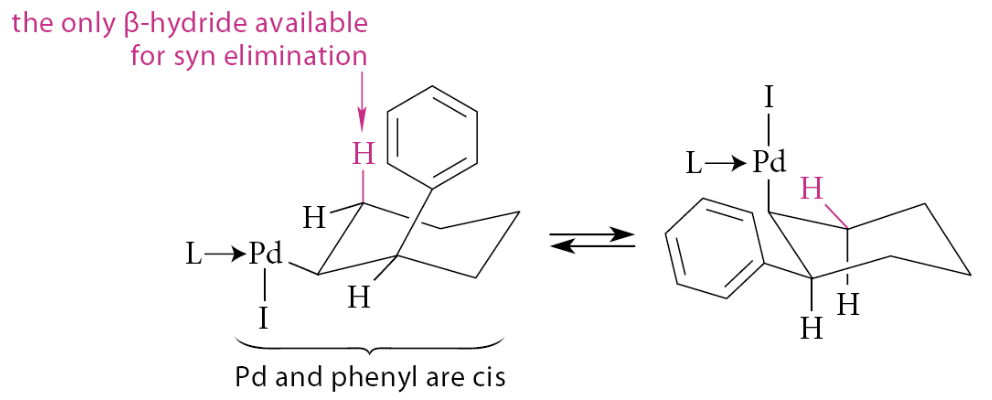
This sort of product is a direct consequence of the stereochemistry of certain steps in the mechanism
The insertion step must occur in a syn manner because the reaction is intramolecular
In the initially formed insertion complex, the Pd and the phenyl group become cis substituents on a cyclohexane ring
What is the Suzuki-Miyaura coupling reaction (Suzuki reaction or Suzuki coupling)?
A Pd(0)-catalyzed process in which an aryl or vinylic boronic acid (a compound of the form RB(OH)2, where R = an aryl or vinylic group) is coupled to an aryl or vinylic iodide or bromide in the presence of a base, which is in many cases aqueous sodium hydroxide or sodium carbonate.
What can the Suzuki-Miyaura coupling reaction be used to prepare?
3 compound types
Biaryls: compounds in which two aryl rings are connected by a σ bond; aryl-substituted alkenes; and conjugated alkenes
The Pd(0) catalyst can be Pd(PPh3)4, the same catalyst used in the Heck reaction, or the Pd(0) can be formed in the reaction flask from Pd(OAc)2, a strategy that is also used in the Heck reaction
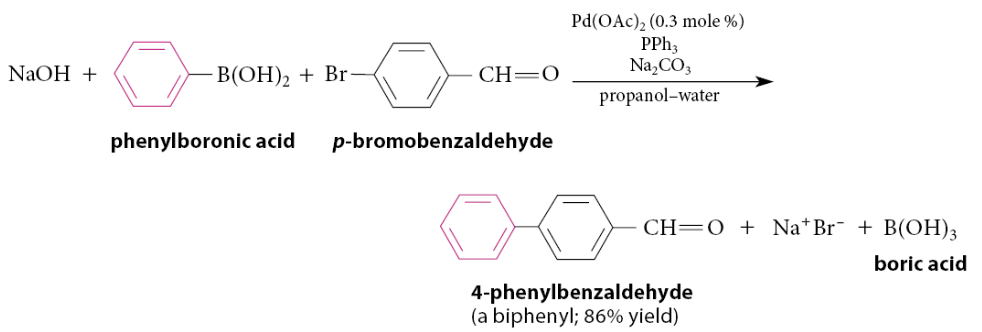
Does the Suzuki-Miyaura coupling reaction have retention?
The coupling occurs with retention of alkene stereochemistry
In this example, this type of compound can be prepared by the Heck reaction. However, the Suzuki coupling avoids issues of regiochemistry that can sometimes occur with the Heck reaction
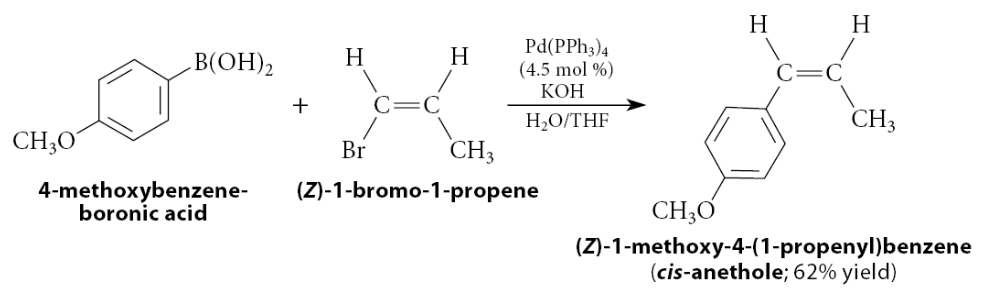
How can you prepare boron derivates?
The reaction of Grignard or organolithium reagent with trimethyl borate
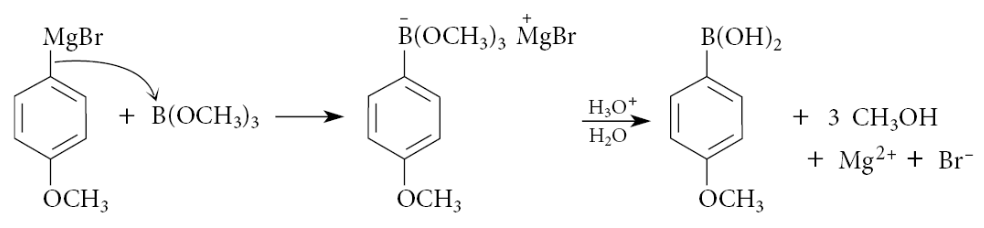
What is a second preparation of vinylic boronic acid derivatives?
A second preparation of vinylic boronic acid derivatives is the hydroboration of 1-alkynes with catecholborane:
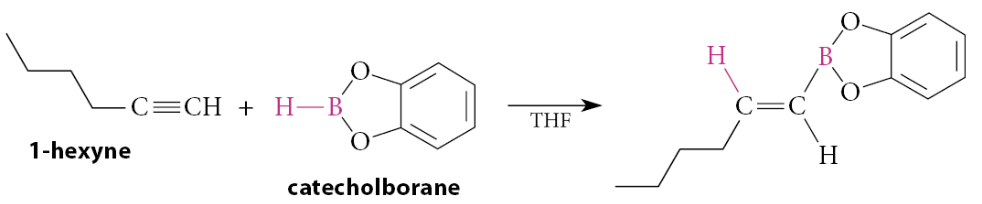
What is the mechanism for the Suzuki-Miyaura coupling reaction?
Ligand dissociation to give a 14e− complex, followed by oxidative addition of the aryl halide
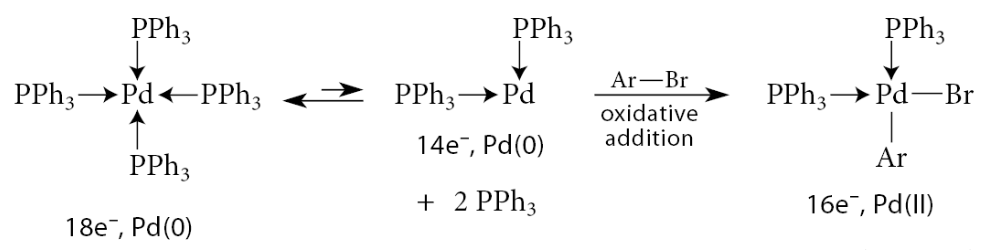
From this point on, the mechanism is not definitively established, but a reasonable sequence involves another ligand substitution in which the base displaces the halide ion:
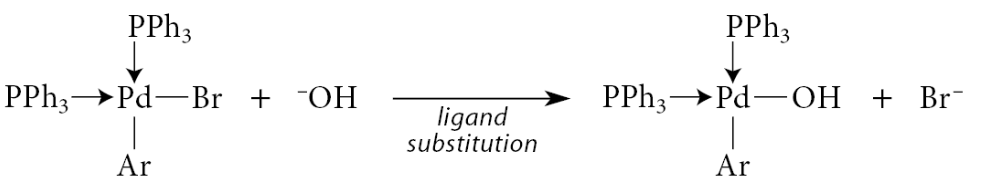
A Lewis acid–base association brings the boron into the coordination sphere of the metal:
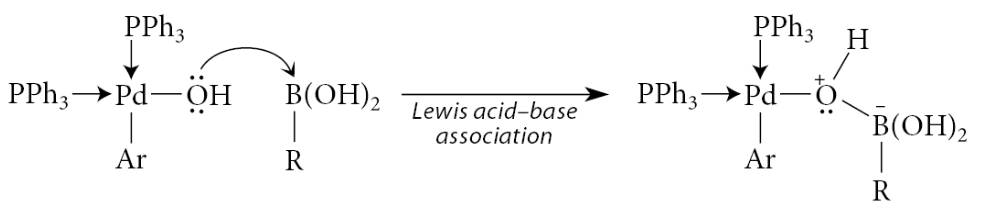
This association results in a formal negative charge on boron. Carbon bears a significant amount of the negative charge in this complex. The Lewis acid–base association of the oxygen with boron endows the carbon in the carbon–boron bond with significant carbanion character. An intramolecular substitution of this “carbon anion,” a strong base, for the weaker base HO—B(OH)2 results in transfer of the R group to the metal. Reductive elimination then gives the coupling product and provides the catalyst for another cycle
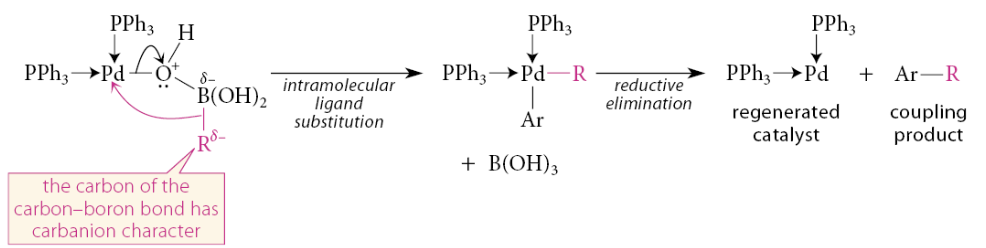
What can certain ruthenium(IV) catalysts cause?
A reaction in which two alkenes are “sliced apart” at their double bonds, and the parts reassembled, to give new alkenes
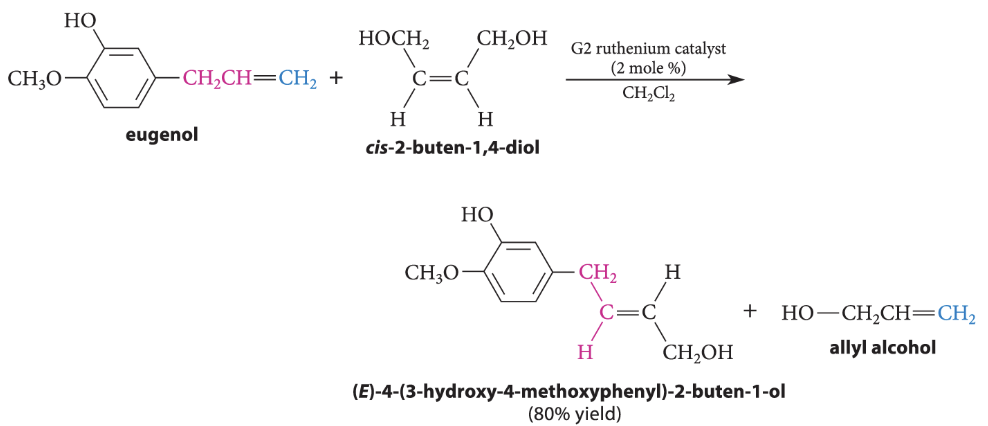
What is alkene metathesis?
The groups at each end of the double bonds are interchanged
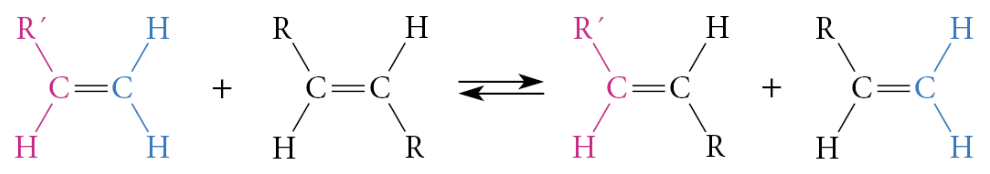
What can happen in a metathesis reaction with 2 unsymmetrical alkenes?
10 alkenes (not counting stereoisomers) can be formed
What are the most widely used laboratory catalysts for alkene metathesis?
The two most widely used laboratory catalysts are the ruthenium-based catalysts G1, which stands for the Grubbs first-generation catalyst, and G2, the Grubbs second-generation catalyst
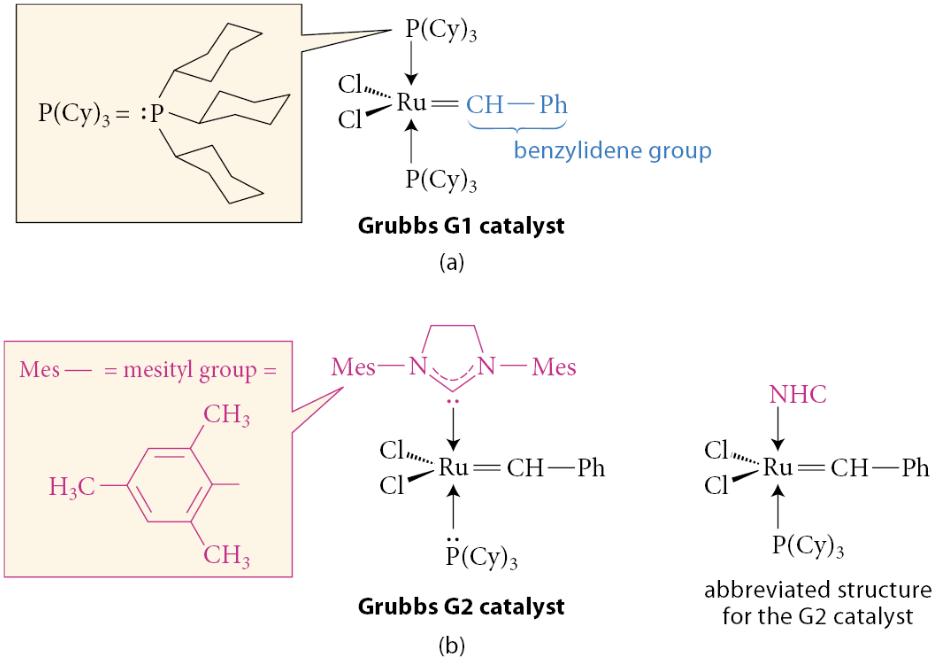
Why does the ruthenium-carbon double bond play an important role in the operation of these catalysts?
The rather unusual NHC ligand in the G2 catalyst, when “dissociated” from the metal, is actually a carbene. Most carbenes are very unstable, but this carbene is stabilized by resonance
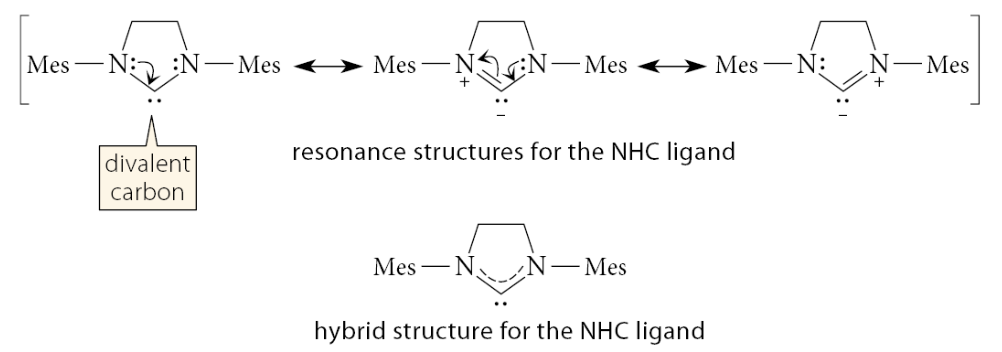
What ligands are NHC, PCy3, chlorides, and benzylidene groups?
The NHC and PCy3 ligands are L-type ligands, the chlorines are X-type ligands, and the benzylidene group is a 2X ligand because it has two bonds to the ruthenium
What can the catalysts do?
The catalysts bring some or all of the possible alkenes into equilibrium. In such cases, the practical use of alkene metathesis requires the application of Le Chatelier’s principle
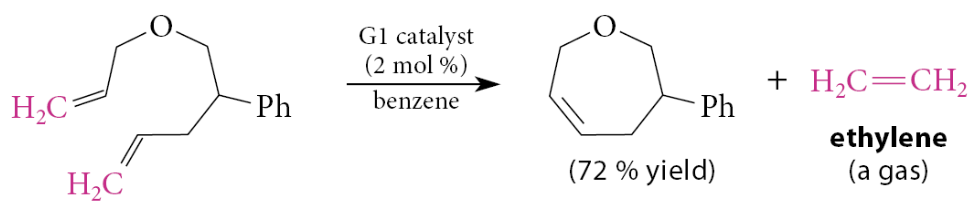
What is one of the most important applications of alkene metathesis?
One of the most important applications of alkene metathesis is for closing rings, and it can be used to close medium- and large-sized rings
In this and many other ring-closing applications, the by-product is ethylene
What is the mechanism for alkene metathesis?
The mechanism of alkene metathesis starts with loss of the PCy3 ligand by ligand dissociation. Because ruthenium in the resulting complex has 14 electrons, it can accept 2 electrons from another ligand, in this case one of the alkenes. Let the alkene RCH=CHR be present in large excess; because of its concentration, it is more likely to interact with the catalyst.
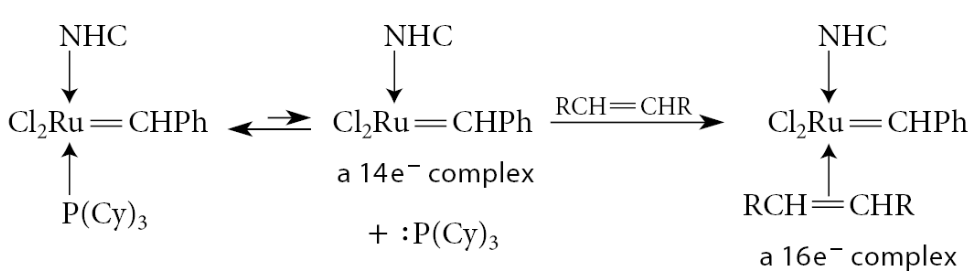
Then follows a key step in alkene metathesis, a cycloaddition to form a metallacycle (a cyclic compound in which the metal occupies a ring position). This reaction is essentially a ligand insertion
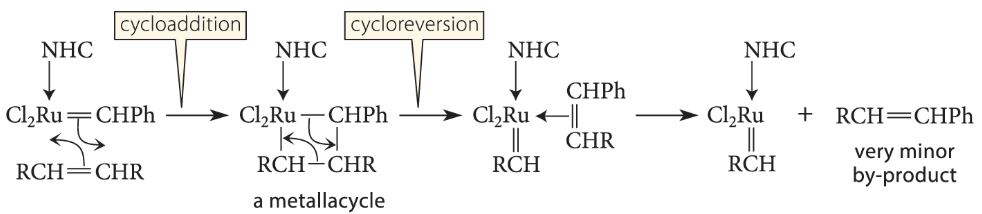
The metallacycle then breaks down in the opposite sense by a cycloreversion (the reverse of a cycloaddition) to give a new alkene, which contains the benzylidene group. This becomes a very minor by-product, because the catalyst (and therefore the benzylidene group) is typically present in 1 or 2 mol % of the reactants. This process leaves the catalyst “primed” with the RCH=group
The cycloaddition–cycloreversion process is now repeated. It is most probable that the process will occur with the alkene. because this alkene is in excess; but the reaction with this alkene results in no change
Occasionally a molecule of the other alkene R′CH=CH2 will bind to the catalyst, and this produces a metathesis product
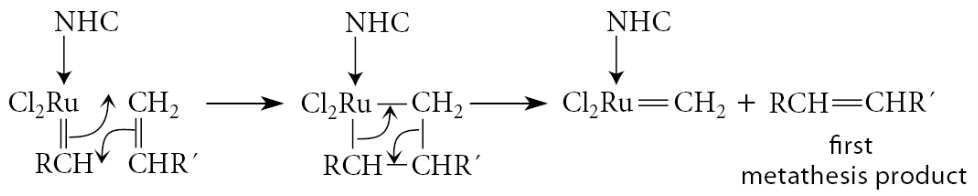
This leaves the catalyst with a =CH2 group. This catalyst molecule is most likely to react with the alkene in excess, and when that happens, the second metathesis product is formed, and the catalyst is again primed with a =CHR group
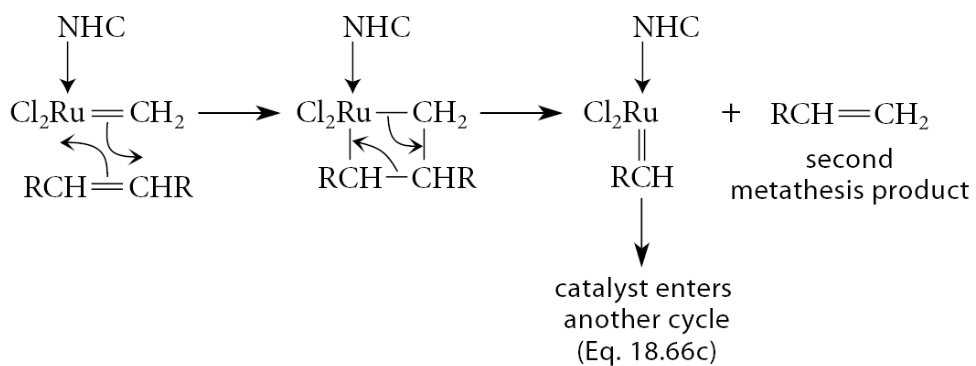
The sequence continues repeatedly until the limiting reactant is exhausted
What is alkene metathesis sensitive to?
The steric environment of the alkene double bonds
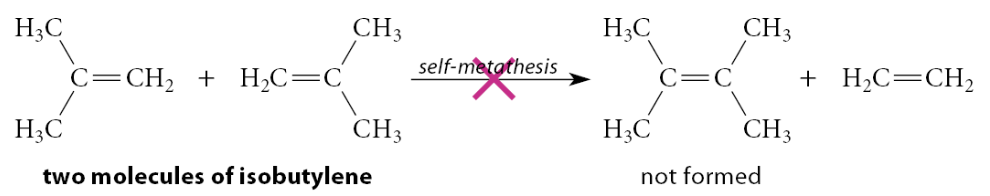
Example:
Methylpropene (isobutylene) does not undergo self-metathesis, presumably because the interaction of two (CH3)2C= fragments with the catalyst results in severe van der Waals repulsions (that is, steric congestion) with the bulky catalyst ligands
What is the Ziegler-Natta catalyst?
A catalyst formed from TiCl3 and (CH3CH2)2AlCL
This catalyzes the polymerization of ethylene and other alkenes at 25 °C and 1 atm pressure
The Ziegler–Natta polymerization of ethylene accounts for most of the polyethylene produced; the resulting high-density polyethylene has different properties from the low-density polyethylene produced by free-radical processes
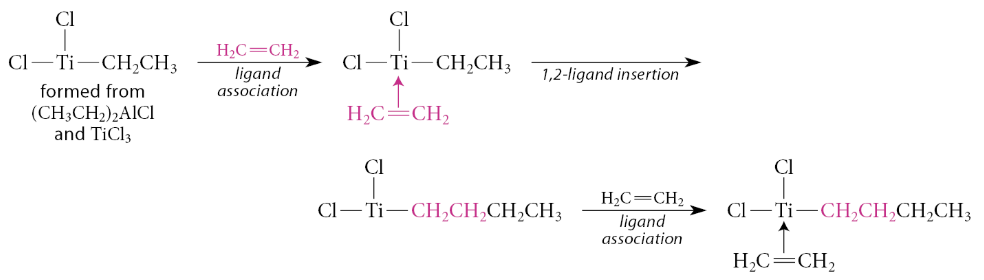
Continuation of the insertion–ligand-association sequence gives the polymer
What is hydroformylation?
Another commercially important process that involves a transition-metal catalyst, in this case a tetracarbonylhydridocobalt(I) catalyst. Propionaldehyde, for example, is produced by the hydroformylation of ethylene
This process involves a 1,2-insertion reaction of ethylene and a 1,1-insertion reaction of carbon monoxide
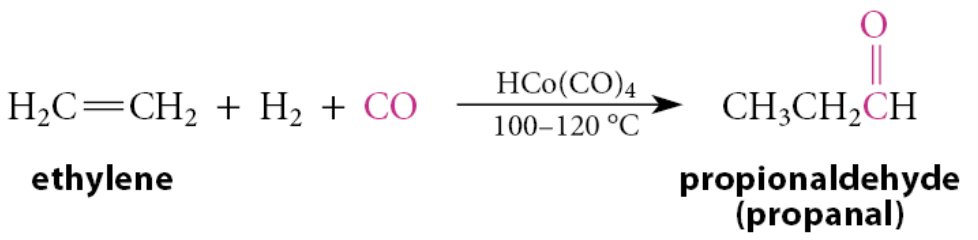
What is the Wilkinson catalyst used for?
The homogeneous catalytic hydrogenation of alkenes using a soluble rhodium(I) catalyst
What is catalytic hydrogenation?
An important reaction that occurs over carbon-supported transition metals such as Ni, Pd, and Pt