Thermal physics
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
Internal energy definition
sum of the randomly distributed kinetic energies and potential energy of the particles in a body.
Key definitions
Particles have random kinetic energy due to random vibrations.
An object has potential energy stored in the bonds between particles.
In an object, the particles have a range of different energies.
Heat
the flow of energy from a higher temperature region to a lower temperature region.
Internal energy of one object decreases
Internal energy of other object(e.g.surroundings) increases
Temperature
The measure of the average random KE of the particles within an object.
How can the internal energy of a system change?
When energy is transferred to it by heating and when work is don't on it.
AND vice versa
Specific Heat Capacity
The heat required to raise the temperature of 1kg of an object by 1°C, without changing state.
ΔQ = mcΔθ
Δθ = change in temperature
c = specific heat capacity
m = mass of the object
q = energy required to increase temp by Δθ Celsius/kelvin
Δθ can be Celsius or kelvin
How to go from kelvin to Celsius
0 kelvin = -273 degrees Celsius
-273
Equilibrium Method
Used when one object is added to another object of different temperature, and there's a different resulting temperature.
Hot object - Temp decreases - (Initial - Final) = Δθ
Cold object - Temp increases - (Final - Initial) = Δθ
use SHC of hot = SHC of cold
SHC rate equations
P = (m/t) x cθ
P = mc x (θ/t)
P = power
m = mass
c = SHC
t = time (seconds)
θ = temp
Heating curve
Where there's a gradient = energy supplied to random KE of particles
where gradient = 0, energy used to break bonds
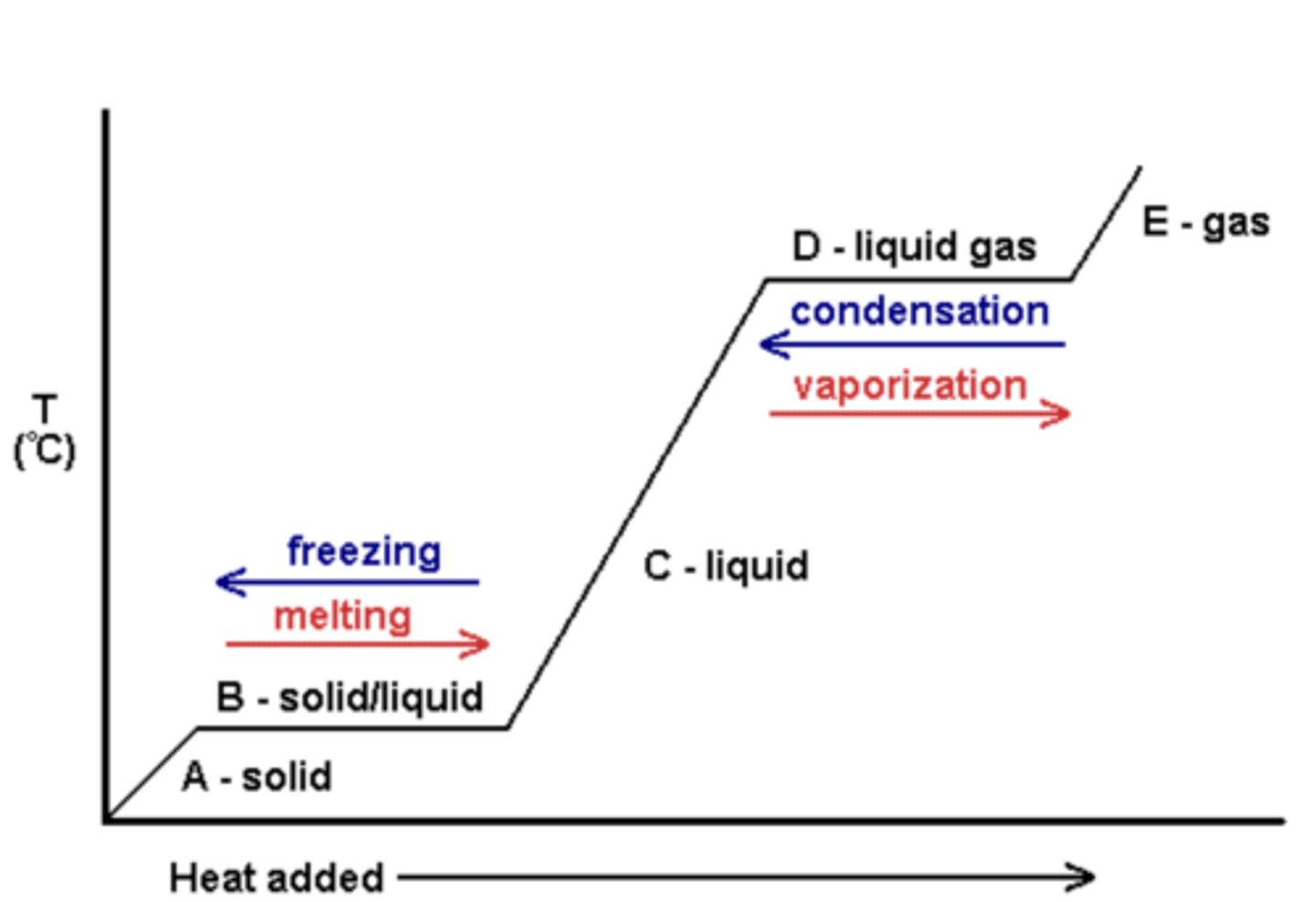
Heating curve NTK
ΔQ = mcΔθ
1/mc = Q/θ, 1/mc = gradient
gradient inversely proportional to SHC.
So if part of graph is steeper, SHC must be less.
For area of vaporisation or melting- energy is used to break bonds.
if one part is longer, the energy to boil/mass is greater
Latent Heat Experiment
Set up two funnels both with 50g of crushed ice.
Beakers underneath both.
put an immersion heater in one and turn it on(will know energy supplied).
measure mass of water in each beaker.
One is a control beaker - how much ice melts due to temperature of the room.
One is due to immersion heater and room.
(ih + r) - (r) = melted ice due to immersion heater
q = Δm x Latent heat
latent heat = energy supplied/mass of water(melted due to immersion heater)
Precautions taken for Latent Heat Experiment
ice crushed into small pieces + immersion heater fully submerged = all of heater is in contact with the ice. Any of the heater not in contact with ice, will heat air, energy dissipated to surroundings.
Using the control set up - finds mass of ice melted due to room temperature.
Latent Heat Formula
Energy = Δm x Latent heat
If a question is asked about how much energy to melt ... from ...
If temp isn't at melting/boiling point.
using SHC formula , Q=mcΔT until melting point
THEN use latent heat formula to find energy needed to melt it.
Latent Heat Definition
The energy required to melt 1kg of a substance, without raising its temperature.
Units for latent heat and specific heat capacity
latent heat = Joules/Kg
Specific heat capacity = Joules/kg(C/K)
SHC and Latent Heat of Ice and Water
SHC of Ice = 2030
SHC of Water = 4200
Latent heat of ice-water = 334000
Assumptions of Kinetic Theory
All collisions are elastic
there is a large number of particles
motions of particles is random
classical laws of physics apply
particles are identical
volume of gas > volume of particles
time of collisions < time between collisions
no external forces on the particles(except gravity)
no intermolecular forces except during collisions.
How to find rms
Square all the values, divide it by the number of values, and then square root that number
Derivation of kinetic theory formula
velocity of particle before colliding with wall = c
velocity of particle after colliding with wall = -c
change in momentum = -2mc
t = 2l /c
force = (-2mc(x)²)/l
p(x) = F/A = F/l² = mc(x)² / l³ = mc(x)²/V - for one particle
for N number of particles = m(c(x)² ..... + c(Nx)²)/V
c(rms,x) = √(c(x)² ..... + c(Nx)²)/N
Nc²(rms,x) = (c(x)² ..... + c(Nx)²)
P(Nx) = (m/v) x N x c²(rms,x)
if motion is radom, magnitude of particles in x,y,z are reoughly the same
c² = c(x)² + c(y)² + c(z)²
c² = 3c(x)²
c²(rms) = 3c(rms,x)²
c²(rms)/3 = c(rms,x)²
P(Nx) = (m/v) x N x c²(rms) x (1/3)
Kinetic theory formula variables
p = pressure
v = volume
N = number of particles
m = mass of a particle
Nm/v = density
c²(rms) - to find speed, you square root it.
Fixed amount of gas, temperature is constant, volume decreases
discuss how the pressure changes
SA of gas reduces, so with the same force pressure will increase, P=F/A
The distance travelled between collisions is reduced , so there are more frequent collisions.
Temp is constant so velocity and force each particle exerts during each collision is constant.
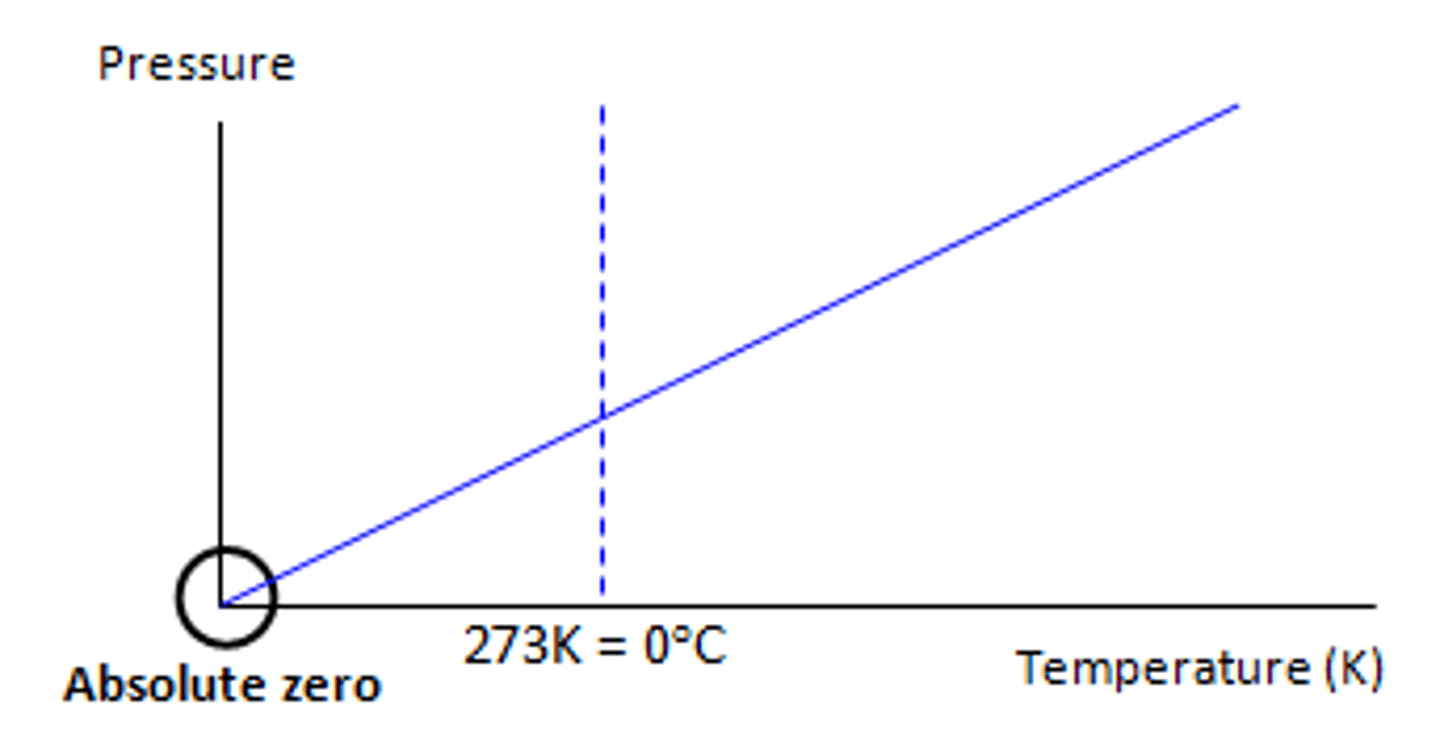
Absolute Temperature
Temperature is a measure of the average kinetic energy of the particles.
At absolute zero, particles would have no kinetic energy, and stop moving and therefore not exert a pressure.
Absolute temperature scale starts at zero.
Example would be Kelvin.
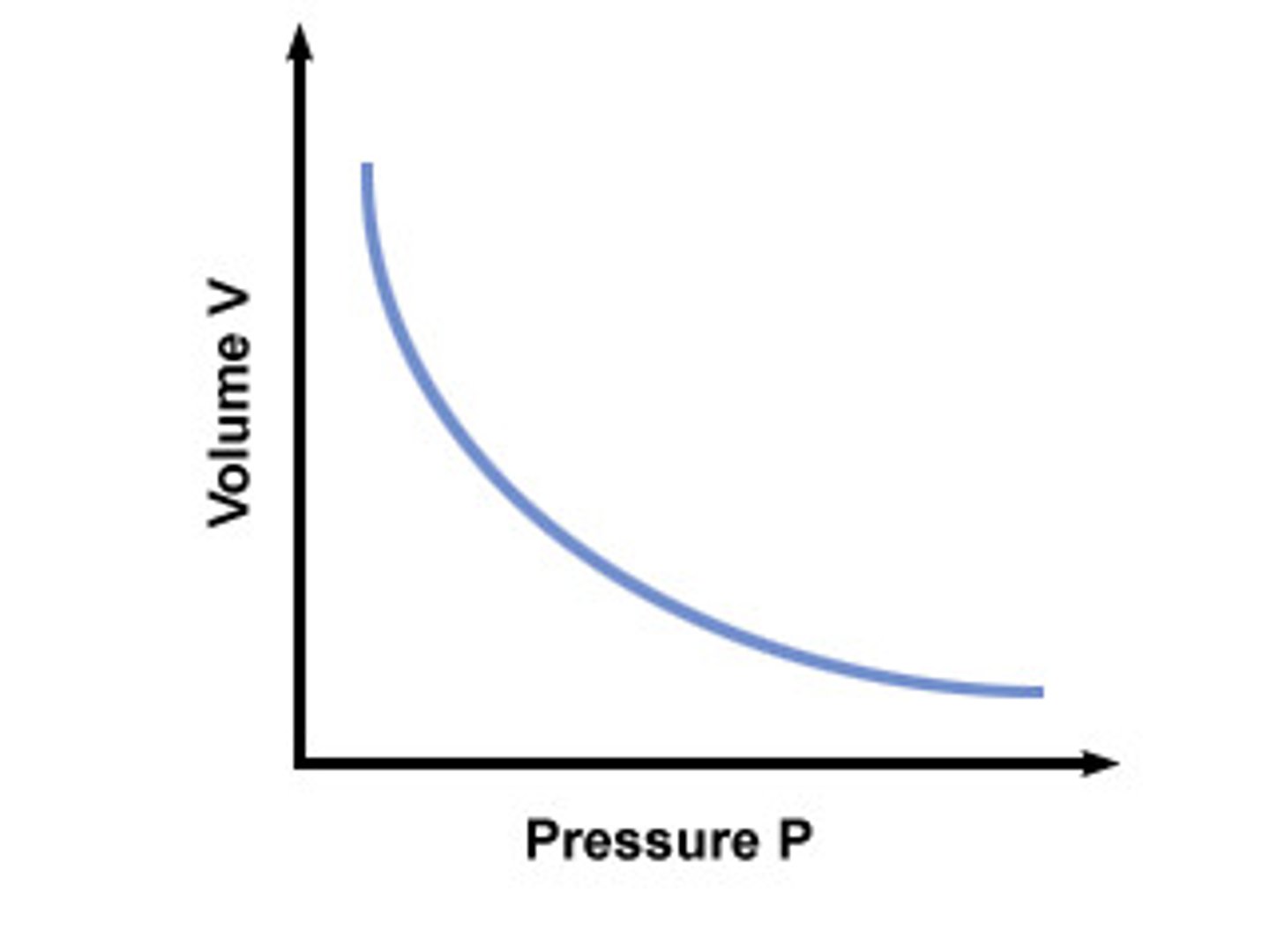
Boyles Law
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.
Pressure Law
For a fixed mass of gas at constant volume the pressure is directly proportional to the absolute temperature.
Charles Law
For a fixed mass of gas at a constant pressure the volume is directly proportional to the absolute temperature.
Combining gas laws
PV/T = constant
P1V1/T1 = P2V2/T2
How to plot graph of variables that are inversely proportional?
One axis - 1/ first variable
Other axis - 2nd variable
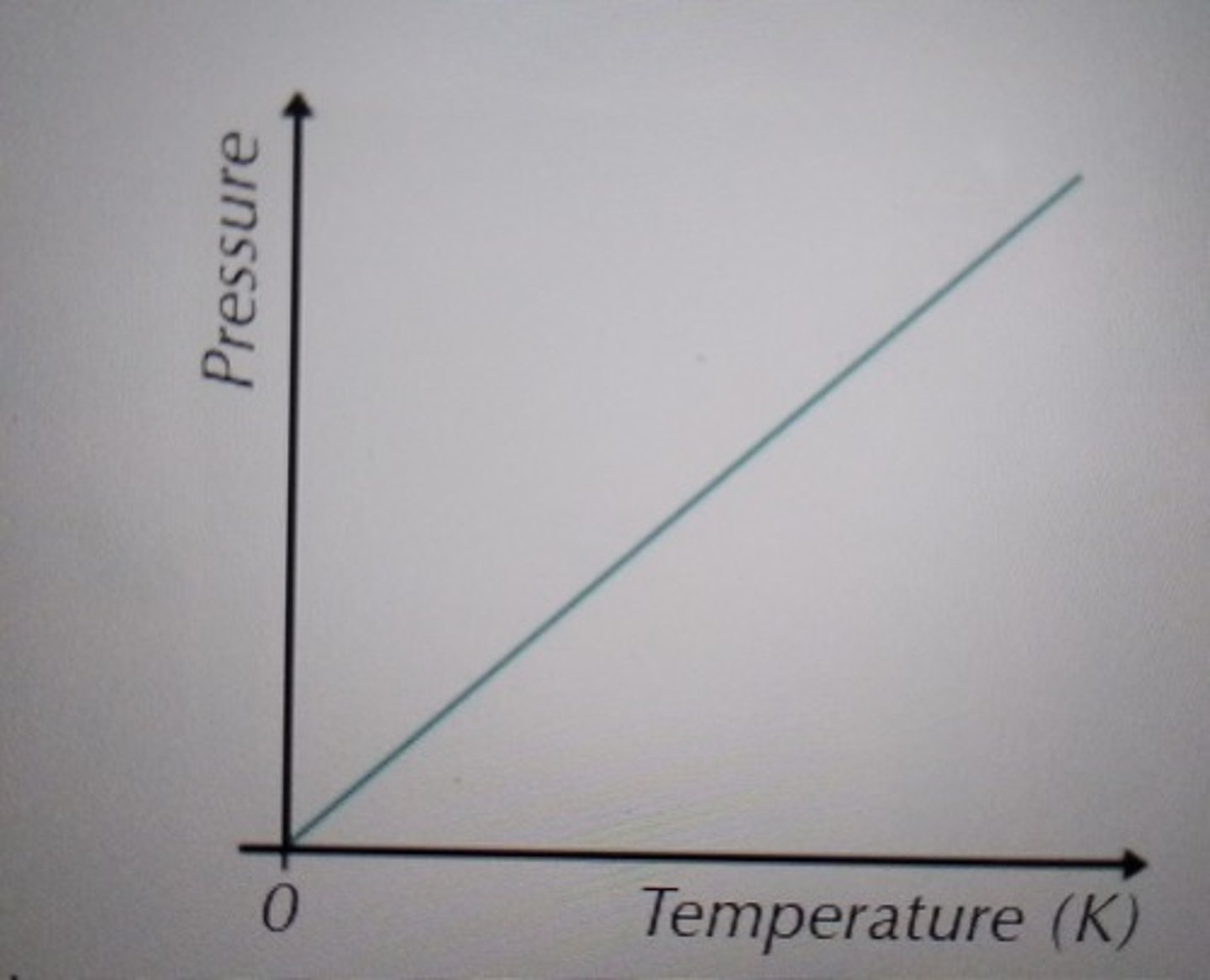
Pressure against absolute temperature (graph)
Pressure against temp in Celsius (graph)
Formulas for ideal gas laws
All equations in Kelvin.
PV = NkT , N = number of particles, k = 1.38 x 10^-23
PV = nRT, n = number of moles, R = 8.31
Equations to do with moles
N = number of particles, n= number of moles, NA = Avogadro's constant, m = mass , Mr = molar mass
N = n/NA
mass of substance = Mr x NA
mass of one particle = Mr / NA
How to find answer to do with change in mass or volume of a system?
have to use
PV = NkT or PV = nRT , use second one as easier numbers
cant use pv/t formula as density changes with change in mass or volume
find mass of new system
subtract mass of old system
thats increase in mass.
How to answer question with two flasks connected. finding mass or volume
Pressure of both is the same.
either equate the equations
or divide one by the other
how to find pressure after of connected vessel
for first flask find pressure after it occupies the entire volume of bath flasks.
then repeat for second flash.
add them together.
average molecular KE formula
1.5kT = 0.5 x m x c^2 rms
m - mass of one particle/molecule
when temp is doubled
you double the kelvin, not celsius
for complicated questions when stuck
find equation for both scenarios and then divide.
1 other formula
k = R x Na(Avogadro's constant)
increase temp...
ke and velocity of particles increase, force exerted increases
frequency of collisions increase due to increased speed,
root mean squared speed
mean squared speed
rms - √c^rms - m/s
ms - c^2rms -m^2/s^2
What's the difference between seatbelt and airbag?
same force
smaller area
higher pressure on parts of body
Total Kinetic energy
Average KE of one particles X number of particles
Ideal Gas
Molecules have negligible volume.
collisions are elastic.
Work done formula
W=PAd
W=P x change in Volume
PV graphs
Work done is area under the graph
Work is only done when volume changes
Work done on or by gas
On gas - compression and energy into gas.
By gas - Expansion and energy away from gas.
negative work done is work done by the gas.
Internal energy formula
ΔU = Q + W
Q - heat supplied to the gas. (Negative means heat is removed from the gas).
W is work done on the gas. (Negative means work done by the gas).
PV graph NTKs
Higher on graph = Higher Temperature
At fixed position and higher temp --- straight line up to T2.
Free to move and heated - Straight line across to T2.
For a fixed piston, that is heated will the gain of thermal energy be the same for a piston that is free to move?
ΔU = Q + W
For fixed piston : Is fixed so doesn't expand so no work is done. So ΔU = Q, which is heat supplied.
For a free piston: is free to move so will expand, when expands work is done by the gas, work done is negative.
ΔU = Q - W
So gain in thermal energy is larger for a fixed piston.
Draw on Graph
Temp from T2-T1 at constant pressure
Temp rise at constant volume
Straight Line across
Straight line up
Why does pressure decrease when there's less particles?
Pressure is due to collisions with walls.
Fewer particles, frequency of collisions with walls decreases.
But because temp is constant, velocity of particles and force exerted remains the same.
so total force exerted reduces.